Novel insights on the pulmonary vascular consequences of COVID-19
- PMID: 32551862
- PMCID: PMC7414237
- DOI: 10.1152/ajplung.00195.2020
Novel insights on the pulmonary vascular consequences of COVID-19
Abstract
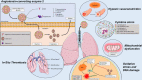
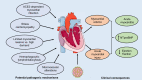
In the last few months, the number of cases of a new coronavirus-related disease (COVID-19) rose exponentially, reaching the status of a pandemic. Interestingly, early imaging studies documented that pulmonary vascular thickening was specifically associated with COVID-19 pneumonia, implying a potential tropism of the virus for the pulmonary vasculature. Moreover, SARS-CoV-2 infection is associated with inflammation, hypoxia, oxidative stress, mitochondrial dysfunction, DNA damage, and lung coagulopathy promoting endothelial dysfunction and microthrombosis. These features are strikingly similar to what is seen in pulmonary vascular diseases. Although the consequences of COVID-19 on the pulmonary circulation remain to be explored, several viruses have been previously thought to be involved in the development of pulmonary vascular diseases. Patients with preexisting pulmonary vascular diseases also appear at increased risk of morbidity and mortality. The present article reviews the molecular factors shared by coronavirus infection and pulmonary vasculature defects, and the clinical relevance of pulmonary vascular alterations in the context of COVID-19.
Keywords: COVID-19; SARS-CoV-1; SARS-CoV-2; coronavirus; pulmonary vascular diseases; vascular remodeling.
Conflict of interest statement
S.P. reports grants from Actelion, grants from AstraZeneca, grants from Resverlogix, outside of the submitted work. M.L. reports personal fees from Air Liquide Health Care, outside the submitted work. S.P. received speaker fees from Actelion Pharmaceuticals and unrestricted grant from Actelion Pharmaceuticals, AstraZeneca (in-kind), Glaxo-Smith-Kline and Resverlogix outside the context of the submitted work. M.L. received speaker fees, and nonfinancial support from Air Liquide Healthcare and SEFAM outside the context of the submitted work. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures
References
-
- Alexander PE, Debono VB, Mammen MJ, Iorio A, Aryal K, Deng D, Brocard E, Alhazzani W. COVID-19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J Clin Epidemiol 123: 120–126, 2020. doi: 10.1016/j.jclinepi.2020.04.016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

