Formulation and investigation of pilocarpine hydrochloride niosomal gels for the treatment of glaucoma: intraocular pressure measurement in white albino rabbits
- PMID: 32551978
- PMCID: PMC8216479
- DOI: 10.1080/10717544.2020.1775726
Formulation and investigation of pilocarpine hydrochloride niosomal gels for the treatment of glaucoma: intraocular pressure measurement in white albino rabbits
Abstract
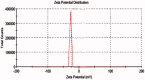
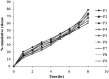
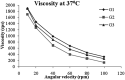
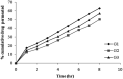
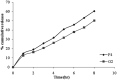
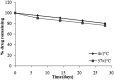
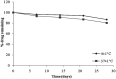
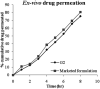
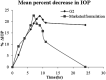
The present study was focused on investigating niosomal gels loaded with cholinergic drug; pilocarpine HCl, for prolonged precorneal residence time and improved bioavailability for glaucoma treatment. Pilocarpine HCl niosomes were prepared using various nonionic surfactants (span 20, span 60 and span 80), in the presence of cholesterol in different molar ratios by ether injection method. The selected formulations were incorporated into carbopol 934 and locust bean gum-based gels. TEM analysis confirmed that niosomes formed were spherical in shape and has a definite internal aqueous space with uniform particle size. Formulation F4 composed of span 60 and cholesterol (1:1) gave the highest entrapment (93.26 ± 1.75%) and slower release results after 8 hours (Q8h = 60.35 ± 1.87%) among other formulations. The in-vitro drug permeation studies showed that there was a prolonged release of drug from niosomal gels as compared to niosomes itself. Considering the in-vitro drug release, niosomal gel formulation G2 was the best among the studied formulations. The release data were fitted to an empirical equation, which indicated that the release follows non-Fickian diffusion mechanism. The stability study revealed that incorporation of niosomes in gel increased their stability than the niosome itself. No signs of redness, inflammation, swelling or increased tear production were observed over the study period for tested formulation by Draize's test. The intraocular pressure (IOP) lowering activity of G2 formulation showed relative bioavailability 2.64 times more than bioavailability of marketed Pilopine HS® gel. These results suggest that the niosomal gels containing pilocarpine HCl are promising ocular carriers for glaucoma treatment.
Keywords: Draize test; IOP; Niosomes; niosomal gel; pilocarpine HCl.
Conflict of interest statement
The authors report no conflicts of interest.
Figures



References
-
- Bayindir ZS, Yuksel N. (2010). Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J Pharm Sci 99:2049–60. - PubMed
-
- Bonomi L, Perfetti S, Noya E, et al. (1978). Experimental corticosteroid ocular hypertension in the rabbit. Albrecht Von Graefes Arch Klin Exp Ophthalmol 209:73–82. - PubMed
-
- Brubaker RF. (2003). Introduction: three targets for glaucoma management. Surv Ophthalmol 48:S1–S2. - PubMed
-
- Carafa M, Santucci E, Alhaique F, et al. (1998). Preparation and properties of new unilamellar non-ionic/ionic surfactant vesicles. Int J Pharm 160:51–9.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
