Chorioallantoic Membrane Tumor Model for Evaluating Oncolytic Viruses
- PMID: 32552215
- PMCID: PMC7585625
- DOI: 10.1089/hum.2020.045
Chorioallantoic Membrane Tumor Model for Evaluating Oncolytic Viruses
Abstract
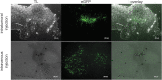
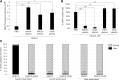
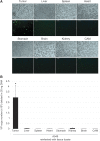
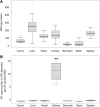
Oncolytic viruses are promising anticancer agents; however, regarding their clinical efficacy, there is still significant scope for improvement. Preclinical in vivo evaluation of oncolytic viruses is mainly based on syngeneic or xenograft tumor models in mice, which is labor-intensive and time-consuming. Currently, a large proportion of developmental work in the research field of oncolytic viruses is directed toward overcoming cellular and noncellular barriers to achieve improved virus delivery to primary tumors and metastases. To evaluate the large number of genetically or chemically modified viruses regarding tumor delivery and biodistribution patterns, it would be valuable to have an in vivo model available that would allow easy screening experiments, that is of higher complexity than monoclonal cell lines, and that could be used as a platform method before confirmatory studies in small and large animals. Based on our data, we believe that the chicken chorioallantoic membrane (CAM) assay is a quick and low-cost high-throughput tumor model system for the in vivo analysis of oncolytic viruses. Here we describe the establishment, careful characterization, and optimization of the CAM model as an in vivo model for the evaluation of oncolytic viruses. We have used human adenovirus type 5 (HAdV-5) as an example for validation but are confident that the model can be used as a test system for replicating viruses of many different virus families. We show that the CAM tumor model enables intratumoral and intravenous virus administration and is a feasible and conclusive model for the analysis of relevant virus-host interactions, biodistribution patterns, and tumor-targeting profiles.
Keywords: adenovirus; biodistribution; chorioallantoic membrane; oncolytic virus; tumor targeting.
Conflict of interest statement
No competing financial interests exist.
Figures

References
-
- Bluming AZ, Ziegler JL. Regression of Burkitt's lymphoma in association with measles infection. Regres Burkitts Lymphoma Assoc Measles Infect 1971;105–106 - PubMed
-
- Mullen JT, Donahue JM, Chandrasekhar S, et al. . Oncolysis by viral replication and inhibition of angiogenesis by a replication-conditional herpes simplex virus that expresses mouse endostatin. Cancer 2004;101:869–877 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
