Event-Free Survival, a Prostate-Specific Antigen-Based Composite End Point, Is Not a Surrogate for Overall Survival in Men With Localized Prostate Cancer Treated With Radiation
- PMID: 32552276
- PMCID: PMC8265328
- DOI: 10.1200/JCO.19.03114
Event-Free Survival, a Prostate-Specific Antigen-Based Composite End Point, Is Not a Surrogate for Overall Survival in Men With Localized Prostate Cancer Treated With Radiation
Abstract
Purpose: Recently, we have shown that metastasis-free survival is a strong surrogate for overall survival (OS) in men with intermediate- and high-risk localized prostate cancer and can accelerate the evaluation of new (neo)adjuvant therapies. Event-free survival (EFS), an earlier prostate-specific antigen (PSA)-based composite end point, may further expedite trial completion.
Methods: EFS was defined as the time from random assignment to the date of first evidence of disease recurrence, including biochemical failure, local or regional recurrence, distant metastasis, or death from any cause, or was censored at the date of last PSA assessment. Individual patient data from trials within the Intermediate Clinical Endpoints in Cancer of the Prostate-ICECaP-database with evaluable PSA and disease follow-up data were analyzed. We evaluated the surrogacy of EFS for OS using a 2-stage meta-analytic validation model by determining the correlation of EFS with OS (patient level) and the correlation of treatment effects (hazard ratios [HRs]) on both EFS and OS (trial level). A clinically relevant surrogacy was defined a priori as an R2 ≥ 0.7.
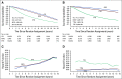
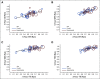
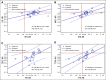
Results: Data for 10,350 patients were analyzed from 15 radiation therapy-based trials enrolled from 1987 to 2011 with a median follow-up of 10 years. At the patient level, the correlation of EFS with OS was 0.43 (95% CI, 0.42 to 0.44) as measured by Kendall's tau from a copula model. At the trial level, the R2 was 0.35 (95% CI, 0.01 to 0.60) from the weighted linear regression of log(HR)-OS on log(HR)-EFS.
Conclusion: EFS is a weak surrogate for OS and is not suitable for use as an intermediate clinical end point to substitute for OS to accelerate phase III (neo)adjuvant trials of prostate cancer therapies for primary radiation therapy-based trials.
Figures
Comment in
-
Socioeconomic Factors, Urological Epidemiology and Practice Patterns.J Urol. 2021 Mar;205(3):920-922. doi: 10.1097/JU.0000000000001552. Epub 2020 Dec 23. J Urol. 2021. PMID: 33356451 No abstract available.
References
-
- Torre LA, Bray F, Siegel RL, et al. : Global cancer statistics, 2012. CA Cancer J Clin 65:87-108, 2015 - PubMed
-
- Siegel RL, Miller KD, Jemal A: Cancer statistics, 2016. CA Cancer J Clin 66:7-30, 2016 - PubMed
-
- Bolla M, Gonzalez D, Warde P, et al. : Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 337:295-300, 1997 - PubMed
-
- Denham JW, Steigler A, Lamb DS, et al. : Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 12:451-459, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

