Red-Light (670 nm) Therapy Reduces Mechanical Sensitivity and Neuronal Cell Death, and Alters Glial Responses after Spinal Cord Injury in Rats
- PMID: 32552352
- PMCID: PMC7585617
- DOI: 10.1089/neu.2020.7066
Red-Light (670 nm) Therapy Reduces Mechanical Sensitivity and Neuronal Cell Death, and Alters Glial Responses after Spinal Cord Injury in Rats
Abstract
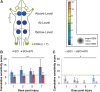
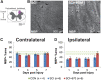
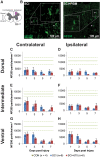
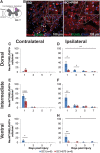
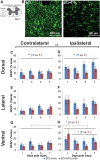
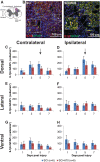
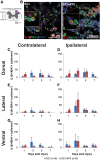
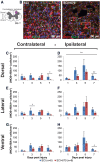
Individuals with spinal cord injury (SCI) often develop debilitating neuropathic pain, which may be driven by neuronal damage and neuroinflammation. We have previously demonstrated that treatment using 670 nm (red) light irradiation alters microglia/macrophage responses and alleviates mechanical hypersensitivity at 7 days post-injury (dpi). Here, we investigated the effect of red light on the development of mechanical hypersensitivity, neuronal markers, and glial response in the subacute stage (days 1-7) following SCI. Wistar rats were subjected to a mild hemi-contusion SCI at vertebra T10 or to sham surgery followed by daily red-light treatment (30 min/day; 670 nm LED; 35 mW/cm2) or sham treatment. Mechanical sensitivity of the rat dorsum was assessed from 1 dpi and repeated every second day. Spinal cords were collected at 1, 3, 5, and 7 dpi for analysis of myelination, neurofilament protein NF200 expression, neuronal cell death, reactive astrocytes (glial fibrillary acidic protein [GFAP]+ cells), interleukin 1 β (IL-1β) expression, and inducible nitric oxide synthase (iNOS) production in IBA1+ microglia/macrophages. Red-light treatment significantly reduced the cumulative mechanical sensitivity and the hypersensitivity incidence following SCI. This effect was accompanied by significantly reduced neuronal cell death, reduced astrocyte activation, and reduced iNOS expression in IBA1+ cells at the level of the injury. However, myelin and NF200 immunoreactivity and IL-1β expression in GFAP+ and IBA1+ cells were not altered by red-light treatment. Thus, red-light therapy may represent a useful non-pharmacological approach for treating pain during the subacute period after SCI by decreasing neuronal loss and modulating the inflammatory glial response.
Keywords: neuroinflammation; neuronal death; neuropathic pain; photobiomodulation; spinal cord injury.
Conflict of interest statement
No competing financial interests exist.
Figures

References
-
- World Health Organization and International Spinal Cord Society. (2013). International perspectives on spinal cord injury. World Health Organization: Geneva, Switzerland.. https://apps.who.int/iris/bitstream/handle/10665/94190/9789241564663_eng...
-
- Turner J.A., Cardenas D.D., Warms C.A., and McClellan C.B. (2001). Chronic pain associated with spinal cord injuries: a community survey. Arch. Phys. Med. Rehabil. 82, 501–509 - PubMed
-
- Pascoal-Faria P., Yalcin N., and Fregni F. (2015). Neural markers of neuropathic pain associated with maladaptive plasticity in spinal cord injury. Pain Pract. 15, 371–377 - PubMed
-
- Finnerup N.B., Norrbrink C., Trok K., Piehl F., Johannesen I.L., Sorensen J.C., Jensen T.S., and Werhagen L. (2014). Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J. Pain 15, 40–48 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

