Economic Burden of Treatment-Resistant Depression in Privately Insured U.S. Patients with Physical Conditions
- PMID: 32552362
- PMCID: PMC10391320
- DOI: 10.18553/jmcp.2020.20017
Economic Burden of Treatment-Resistant Depression in Privately Insured U.S. Patients with Physical Conditions
Abstract
Background: Little is known about the economic burden of treatment-resistant depression (TRD) in patients with physical conditions.
Objective: To assess health care resource utilization (HRU) and costs, work loss days, and related costs in patients with TRD and physical conditions versus patients with the same conditions and non-TRD major depressive disorder (MDD) or without MDD.
Methods: Adults aged < 65 years with MDD treated with antidepressants were identified in the OptumHealth Care Solutions database (July 2009-March 2017). Patients who received a diagnosis of MDD and initiated a third antidepressant regimen (index date) after 2 regimens of adequate dose and duration were defined as having TRD. Patients with non-TRD MDD and without MDD were assigned a random index date. Patients with < 6 months of continuous health plan eligibility pre- or post-index; a diagnosis of psychosis, schizophrenia, bipolar disorder/mania, dementia, and developmental disorders; and/or no baseline physical conditions (cardiovascular, metabolic, and respiratory disease or cancer) were excluded. Patients with TRD were matched 1:1 to each of the non-TRD MDD and non-MDD cohorts based on propensity scores. Per patient per year HRU, costs, and work loss outcomes were compared up to 24 months post-index date using negative binominal and ordinary least square regressions.
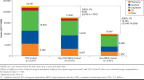
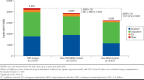
Results: A total of 2,317 patients with TRD (mean age, 47.6 years; 63.1%, female; mean follow-up, 19.7 months) had ≥ 1 co-occurring key physical condition (cardiovascular, 52.5%; metabolic, 48.2%; respiratory, 16.4%; and cancer, 9.5%). Relative to non-TRD MDD and non-MDD cohorts, respectively, patients with TRD had 46% and 235% more inpatient admissions, 28% and 128% more emergency department visits, and 53% and 155% more outpatient visits (all P < 0.05). Health care costs were $22,541 in the TRD cohort, $17,450 in the non-TRD MDD cohort, and $10,047 in the non-MDD cohort, yielding cost differences of $5,091 (vs. non-TRD MDD) and $12,494 (vs. non-MDD; all P < 0.01). In patients with work loss data available (n = 278/cohort), those with TRD had 2.0 and 2.9 times more work loss as well as $8,676 and $10,323 higher work loss costs relative to those with non-TRD MDD and without MDD, respectively (all P < 0.001).
Conclusions: In patients with physical conditions, those with TRD had higher HRU and health care costs, work loss days, and associated costs compared with non-TRD MDD and non-MDD cohorts.
Disclosures: This study was sponsored by Janssen Scientific Affairs (JSA), which was involved in all aspects of the research, including the design of the study; the collection, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication. Joshi and Daly are employed by JSA. Zhdanava, Pilon, Rossi, Morrison, and Lefebvre are employees of Analysis Group, which received funding from JSA for conducting this study and has received consulting fees from Novartis Pharmaceuticals and GSK, unrelated to this study. Kuvadia is employed by Integrated Resources, which has provided research services to JSA unrelated to this study; Joshi reports past employment by and stock ownership in Johnson & Johnson; Nelson reports advisory board, data and safety monitoring board, and consulting fees from Assurex, Eisai, FSV-7, JSA, Lundbeck, Otsuka, and Sunovion and royalties from UpToDate, unrelated to this study. This work was presented at AMCP Nexus 2019, held in National Harbor, MD, from October 29 to November 1, 2019.
Conflict of interest statement
This study was sponsored by Janssen Scientific Affairs (JSA), which was involved in all aspects of the research, including the design of the study; the collection, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication. Joshi and Daly are employed by JSA. Zhdanava, Pilon, Rossi, Morrison, and Lefebvre are employees of Analysis Group, which received funding from JSA for conducting this study and has received consulting fees from Novartis Pharmaceuticals and GSK, unrelated to this study. Kuvadia is employed by Integrated Resources, which has provided research services to JSA unrelated to this study; Joshi reports past employment by and stock ownership in Johnson & Johnson; Nelson reports advisory board, data and safety monitoring board, and consulting fees from Assurex, Eisai, FSV-7, JSA, Lundbeck, Otsuka, and Sunovion and royalties from UpToDate, unrelated to this study.
This work was presented at AMCP Nexus 2019, held in National Harbor, MD, from October 29 to November 1, 2019.
Figures


References
-
- Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication No. PEP19-5068. NSDUH Series H-54. August 2019. Available at: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNatio.... Accessed June 3, 2020.
-
- National Institute of Mental Health. Major depression. February 2019. Available at: https://www.nimh.nih.gov/health/statistics/major-defrpression.shtml. Accessed June 3, 2020.
-
- Joshi K, Zhdanava M, Pilon D, Lefebvre P, Sheehan J. Health care use and associated cost among patients with treatment-resistant depression across payers: a comprehensive analysis. Poster presented at: 2019 AMCP Annual Meeting; March 25-28, 2019; San Diego, CA. Available at: https://www.jmcp.org/doi/pdf/10.18553/jmcp.2019.25.issue-3-a. Accessed June 10, 2020. - DOI
-
- Gaynes B, Asher G, Gartlehner G, et al. Definition of treatment-resistant depression in the Medicare population. February 9, 2019. Available at: https://www.ncbi.nlm.nih.gov/books/NBK526366/. Accessed June 3, 2020. - PubMed
-
- Amos TB, Tandon N, Lefebvre P, et al. Direct and indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a U.S. commercial claims database. J Clin Psychiatry. 2018;79(2):17m11725. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

