Determining the Stoichiometry of Small Protein Oligomers Using Steady-State Fluorescence Anisotropy
- PMID: 32553128
- PMCID: PMC7335908
- DOI: 10.1016/j.bpj.2020.05.025
Determining the Stoichiometry of Small Protein Oligomers Using Steady-State Fluorescence Anisotropy
Abstract
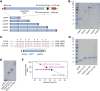
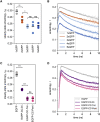
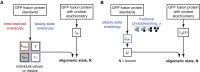
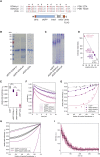
A large fraction of soluble and membrane-bound proteins exists as non-covalent dimers, trimers, and higher-order oligomers. The experimental determination of the oligomeric state or stoichiometry of proteins remains a nontrivial challenge. In one approach, the protein of interest is genetically fused to green fluorescent protein (GFP). If a fusion protein assembles into a non-covalent oligomeric complex, exciting their GFP moiety with polarized fluorescent light elicits homotypic Förster resonance energy transfer (homo-FRET), in which the emitted radiation is partially depolarized. Fluorescence depolarization is associated with a decrease in fluorescence anisotropy that can be exploited to calculate the oligomeric state. In a classical approach, several parameters obtained through time-resolved and steady-state anisotropy measurements are required for determining the stoichiometry of the oligomers. Here, we examined novel approaches in which time-resolved measurements of reference proteins provide the parameters that can be used to interpret the less expensive steady-state anisotropy data of candidates. In one approach, we find that using average homo-FRET rates (kFRET), average fluorescence lifetimes (τ), and average anisotropies of those fluorophores that are indirectly excited by homo-FRET (rET) do not compromise the accuracy of calculated stoichiometries. In the other approach, fractional photobleaching of reference oligomers provides a novel parameter a whose dependence on stoichiometry allows one to quantitatively interpret the increase of fluorescence anisotropy seen after photobleaching the candidates. These methods can at least reliably distinguish monomers from dimers and trimers.
Copyright © 2020 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Goodsell D.S., Olson A.J. Structural symmetry and protein function. Annu. Rev. Biophys. Biomol. Struct. 2000;29:105–153. - PubMed
-
- Neumann J., Klein N., Schneider D. Folding energetics and oligomerization of polytopic α-helical transmembrane proteins. Arch. Biochem. Biophys. 2014;564:281–296. - PubMed
-
- Ali M.H., Imperiali B. Protein oligomerization: how and why. Bioorg. Med. Chem. 2005;13:5013–5020. - PubMed
-
- Gell D.A., Grant R.P., Mackay J.P. The detection and quantitation of protein oligomerization. In: Matthews J.M., editor. Protein Dimerization and Oligomerization in Biology. Springer; 2012. pp. 19–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

