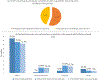
Under-Reporting and Under-Representation of Racial/Ethnic Minorities in Major Atrial Fibrillation Clinical Trials
- PMID: 32553226
- PMCID: PMC7531872
- DOI: 10.1016/j.jacep.2020.03.001
Under-Reporting and Under-Representation of Racial/Ethnic Minorities in Major Atrial Fibrillation Clinical Trials
Conflict of interest statement
Figures
References
-
- January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–132. - PubMed
-
- United States Census Bureau QuickFacts. 2018. Available at https://www.census.gov/quickfacts/fact/table/US/PST045218. Accessed December 15, 2019
-
- United States Census Bureau. US Population - 1940 to 2010. 2012. Available at https://www.census.gov/newsroom/cspan/1940census/CSPAN_1940slides.pdf. Accessed December 15, 2019.
-
- Food and Drug Administration Office of Minority Health. Clinical Trial Diversity Stakeholder Communications Toolkit. 2016. Available at https://www.census.gov/quickfacts/fact/table/US/PST045218. Accessed December 15, 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical