Coronary 18F-Sodium Fluoride Uptake Predicts Outcomes in Patients With Coronary Artery Disease
- PMID: 32553260
- PMCID: PMC7380446
- DOI: 10.1016/j.jacc.2020.04.046
Coronary 18F-Sodium Fluoride Uptake Predicts Outcomes in Patients With Coronary Artery Disease
Abstract
Background: Reliable methods for predicting myocardial infarction in patients with established coronary artery disease are lacking. Coronary 18F-sodium fluoride (18F-NaF) positron emission tomography (PET) provides an assessment of atherosclerosis activity.
Objectives: This study assessed whether 18F-NaF PET predicts myocardial infarction and provides additional prognostic information to current methods of risk stratification.
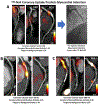
Methods: Patients with known coronary artery disease underwent 18F-NaF PET computed tomography and were followed up for fatal or nonfatal myocardial infarction over 42 months (interquartile range: 31 to 49 months). Total coronary 18F-NaF uptake was determined by the coronary microcalcification activity (CMA).
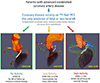
Results: In a post hoc analysis of data collected for prospective observational studies, the authors studied 293 study participants (age: 65 ± 9 years; 84% men), of whom 203 (69%) showed increased coronary 18F-NaF activity (CMA >0). Fatal or nonfatal myocardial infarction occurred only in patients with increased coronary 18F-NaF activity (20 of 203 with a CMA >0 vs. 0 of 90 with a CMA of 0; p < 0.001). On receiver operator curve analysis, fatal or nonfatal myocardial infarction prediction was highest for 18F-NaF CMA, outperforming coronary calcium scoring, modified Duke coronary artery disease index and Reduction of Atherothrombosis for Continued Health (REACH) and Secondary Manifestations of Arterial Disease (SMART) risk scores (area under the curve: 0.76 vs. 0.54, 0.62, 0.52, and 0.54, respectively; p < 0.001 for all). Patients with CMA >1.56 had a >7-fold increase in fatal or nonfatal myocardial infarction (hazard ratio: 7.1; 95% confidence interval: 2.2 to 25.1; p = 0.003) independent of age, sex, risk factors, segment involvement and coronary calcium scores, presence of coronary stents, coronary stenosis, REACH and SMART scores, the Duke coronary artery disease index, and recent myocardial infarction.
Conclusions: In patients with established coronary artery disease, 18F-NaF PET provides powerful independent prediction of fatal or nonfatal myocardial infarction.
Keywords: (18)F-NaF PET; coronary artery disease; coronary computed tomography; coronary event risk prediction; myocardial infarction.
Copyright © 2020 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
18F-Sodium Fluoride PET Imaging Passes an Important Milestone Toward Noninvasive Prediction of Clinical Events.J Am Coll Cardiol. 2020 Jun 23;75(24):3075-3077. doi: 10.1016/j.jacc.2020.04.047. J Am Coll Cardiol. 2020. PMID: 32553261 Free PMC article. No abstract available.
References
-
- Dorresteijn JA, Visseren FL, Wassink AM et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease:the SMART risk score. Heart 2013;99:866–72. - PubMed
-
- Arbab-Zadeh A, Fuster V. From Detecting the Vulnerable Plaque to Managing the Vulnerable Patient: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;74:1582–1593. - PubMed
-
- Dweck MR, Chow MW, Joshi NV et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol 2012;59:1539–48. - PubMed
-
- Joshi NV, Vesey AT,Williams MC et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705–13. - PubMed
-
- Lee JM, Bang JI, Koo BK et al. Clinical Relevance of (18)F-Sodium-Fluoride Positron-Emission Tomography in Noninvasive Identification of High-Risk-Plaque in Patients With Coronary Artery Disease. Circ Cardiovasc Imaging 2017;10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0701127/MRC_/Medical Research Council/United Kingdom
- MR/T005459/1/MRC_/Medical Research Council/United Kingdom
- FS/11/014/BHF_/British Heart Foundation/United Kingdom
- RE/18/5/34216/BHF_/British Heart Foundation/United Kingdom
- WT103782AIA/WT_/Wellcome Trust/United Kingdom
- FS/14/78/31020/BHF_/British Heart Foundation/United Kingdom
- CH/09/002/BHF_/British Heart Foundation/United Kingdom
- R01 HL135557/HL/NHLBI NIH HHS/United States
- RG/16/10/32375/BHF_/British Heart Foundation/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- CH/09/002/26360/BHF_/British Heart Foundation/United Kingdom

