Intussusception in children aged under two years in India: Retrospective surveillance at nineteen tertiary care hospitals
- PMID: 32553492
- PMCID: PMC7528221
- DOI: 10.1016/j.vaccine.2020.04.059
Intussusception in children aged under two years in India: Retrospective surveillance at nineteen tertiary care hospitals
Abstract
Objective: Intussusception has been linked with rotavirus vaccine (RVV) as a rare adverse reaction. In view of limited background data on intussusception in India and in preparation for RVV introduction, a surveillance network was established to document the epidemiology of intussusception cases in Indian children.
Methods: Intussusception in children 2-23 months were documented at 19 nationally representative sentinel hospitals through a retrospective surveillance for 69 months (July 2010 to March 2016). For each case clinical, hospital course, treatment and outcome data were collected.
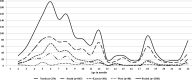
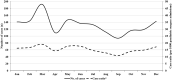
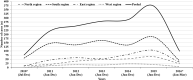
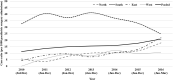
Results: Among the 1588 intussusception cases, 54.5% were from South India and 66.3% were boys. The median age was 8 months (IQR 6, 12) with 34.6% aged 2-6 months. Seasonal variation with higher cases were documented during March-June period. The most common symptoms and signs were vomiting (63.4%), bloody stool (49.1%), abdominal pain (46.9%) and excessive crying (42.8%). The classical triad (vomiting, abdominal pain, and blood in stools) was observed in 25.6% cases. 96.4% cases were diagnosed by ultrasound with ileocolic location as the commonest (85.3%). Management was done by reduction (50.8%) and surgery (41.1%) and only 1% of the patients' died. 91.1% cases met Brighton criteria level 1 and 3.3% Level 2. Between 2010 and 2015, the case load and case ratio increased across all regions.
Conclusion: Intussusception cases have occurred in children across all parts of the country, with low case fatality in the settings studied. The progressive rise cases could indicate an increasing awareness and availability of diagnostic facilities.
Keywords: Children; Epidemiology; India; Intussusception; Retrospective surveillance.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Bines J.E., Kohl K.S., Forster J., Zanardi L.R., Davis R.L., Hansen J. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22(5–6):569–574. - PubMed
-
- Bines JE, Ivanoff B. Acute intussusception in infants and children: Incidence, clinical presentation and management: a global perspective [Internet]. World Health Organisation; 2002 [cited 2017 May 10]. Available from: http://apps.who.int/iris/bitstream/handle/10665/67720/WHO_V-B_02.19_eng.....
-
- Murphy T.V., Gargiullo P.M., Massoudi M.S., Nelson D.B., Jumaan A.O., Okoro C.A. Intussusception among Infants Given an Oral Rotavirus Vaccine. N Engl J Med. 2001;344(8):564–572. - PubMed
-
- Patel M.M., Haber P., Baggs J., Zuber P., Bines J.E., Parashar U.D. Intussusception and rotavirus vaccination: a review of the available evidence. Expert Rev Vaccines. 2009;8(11):1555–1564. - PubMed
-
- World Health Organization Rotavirus vaccines. Releve Epidemiol Hebd. 2007;82(32):285–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

