Tocilizumab Treatment for Cytokine Release Syndrome in Hospitalized Patients With Coronavirus Disease 2019: Survival and Clinical Outcomes
- PMID: 32553536
- PMCID: PMC7831876
- DOI: 10.1016/j.chest.2020.06.006
Tocilizumab Treatment for Cytokine Release Syndrome in Hospitalized Patients With Coronavirus Disease 2019: Survival and Clinical Outcomes
Abstract
Background: Tocilizumab, an IL-6 receptor antagonist, can be used to treat cytokine release syndrome (CRS), with observed improvements in a coronavirus disease 2019 (COVID-19) case series.
Research question: The goal of this study was to determine if tocilizumab benefits patients hospitalized with COVID-19.
Study design and methods: This observational study of consecutive COVID-19 patients hospitalized between March 10, 2020, and March 31, 2020, and followed up through April 21, 2020, was conducted by chart review. Patients were treated with tocilizumab using an algorithm that targeted CRS. Survival and mechanical ventilation (MV) outcomes were reported for 14 days and stratified according to disease severity designated at admission (severe, ≥ 3 L supplemental oxygen to maintain oxygen saturation > 93%). For tocilizumab-treated patients, pre/post analyses of clinical response, biomarkers, and safety outcomes were assessed. Post hoc survival analyses were conducted for race/ethnicity.
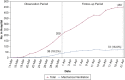
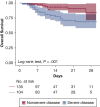
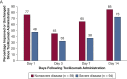
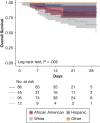
Results: Among the 239 patients, median age was 64 years; 36% and 19% were black and Hispanic, respectively. Hospital census increased exponentially, yet MV census did not. Severe disease was associated with lower survival (78% vs 93%; P < .001), greater proportion requiring MV (44% vs 5%; P < .001), and longer median MV days (5.5 vs 1.0; P = .003). Tocilizumab-treated patients (n = 153 [64%]) comprised 90% of those with severe disease; 44% of patients with nonsevere disease received tocilizumab for evolving CRS. Tocilizumab-treated patients with severe disease had higher admission levels of high-sensitivity C-reactive protein (120 vs 71 mg/L; P < .001) and received tocilizumab sooner (2 vs 3 days; P < .001), but their survival was similar to that of patients with nonsevere disease (83% vs 91%; P = .11). For tocilizumab-treated patients requiring MV, survival was 75% (95% CI, 64-89). Following tocilizumab treatment, few adverse events occurred, and oxygenation and inflammatory biomarkers (eg, high-sensitivity C-reactive protein, IL-6) improved; however, D-dimer and soluble IL-2 receptor (also termed CD25) levels increased significantly. Survival in black and Hispanic patients, after controlling for age, was significantly higher than in white patients (log-rank test, P = .002).
Interpretation: A treatment algorithm that included tocilizumab to target CRS may influence MV and survival outcomes. In tocilizumab-treated patients, oxygenation and inflammatory biomarkers improved, with higher than expected survival. Randomized trials must confirm these findings.
Keywords: COVID-19; cytokine release syndrome; disease severity; mechanical ventilation; survival; tocilizumab.
Published by Elsevier Inc.
Figures

Comment in
-
Questioning Tocilizumab Use in Hospitalized Patients With Coronavirus Disease 2019.Chest. 2021 May;159(5):2115-2116. doi: 10.1016/j.chest.2020.11.069. Chest. 2021. PMID: 33965141 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

