A potent antagonist antibody targeting connexin hemichannels alleviates Clouston syndrome symptoms in mutant mice
- PMID: 32553574
- PMCID: PMC7378960
- DOI: 10.1016/j.ebiom.2020.102825
A potent antagonist antibody targeting connexin hemichannels alleviates Clouston syndrome symptoms in mutant mice
Abstract
Background: Numerous currently incurable human diseases have been causally linked to mutations in connexin (Cx) genes. In several instances, pathological mutations generate abnormally active Cx hemichannels, referred to also as "leaky" hemichannels. The goal of this study was to assay the in vivo efficacy of a potent antagonist antibody targeting Cx hemichannels.
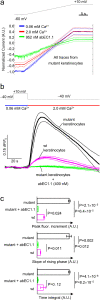
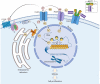
Methods: We employed the antibody to treat Cx30A88V/A88V adult mutant mice, the only available animal model of Clouston syndrome, a rare orphan disease caused by Cx30 p.A88V leaky hemichannels. To gain mechanistic insight into antibody action, we also performed patch clamp recordings, Ca2+ imaging and ATP release assay in vitro.
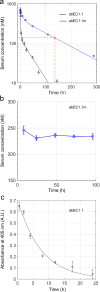
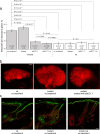
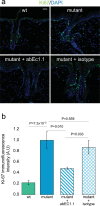
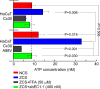
Findings: Two weeks of antibody treatment sufficed to repress cell hyperproliferation in skin and reduce hypertrophic sebaceous glands (SGs) to wild type (wt) levels. These effects were obtained whether mutant mice were treated topically, by application of an antibody cream formulation, or systemically, by intraperitoneal antibody injection. Experiments with mouse primary keratinocytes and HaCaT cells revealed the antibody blocked Ca2+ influx and diminished ATP release through leaky Cx30 p.A88V hemichannels.
Interpretation: Our results show anti-Cx antibody treatment was effective in vivo and sufficient to counteract the effects of pathological connexin expression in Cx30A88V/A88V mice. In vitro experiments suggest antibodies gained control over leaky hemichannels and contributed to restoring epidermal homeostasis. Therefore, regulating cell physiology by antibodies targeting the extracellular domain of Cxs may enforce an entirely new therapeutic strategy. These findings support the further development of antibodies as drugs to address unmet medical needs for Cx-related diseases. FUND: Fondazione Telethon, GGP19148; University of Padova, SID/BIRD187130; Consiglio Nazionale delle Ricerche, DSB.AD008.370.003\TERABIO-IBCN; National Science Foundation of China, 31770776; Science and Technology Commission of Shanghai Municipality, 16DZ1910200.
Keywords: ATP; Antibody drug discovery; Calcium; Epidermis; Genodermatosis; Sebocytes.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Drs. F. Mammano, G. Yang and F. Zonta report a patent: “Fully human antibody specifically inhibiting connexin 26”, Inventors: Qu Z, Yang G, Mammano F, Zonta F, International application number: PCT/CN2016/109847, pending to ShanghaiTech University; and a patent: “Composition and Methods to treat Ectodermal Dysplasia 2, Clouston Type”, Inventors: Mammano F, Yang G, Zonta F, International Application No.: PCT/CN2019/088689, International Filing Date: 2019-05-28, pending to ShanghaiTech University. All other Authors have nothing to declare.
Figures

Comment in
-
Connexin hemichannel inhibition improves skin pathology in Clouston syndrome mice.EBioMedicine. 2020 Jul;57:102856. doi: 10.1016/j.ebiom.2020.102856. Epub 2020 Jul 3. EBioMedicine. 2020. PMID: 32629388 Free PMC article. No abstract available.
Similar articles
-
Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity.Hum Mol Genet. 2004 Aug 15;13(16):1703-14. doi: 10.1093/hmg/ddh191. Epub 2004 Jun 22. Hum Mol Genet. 2004. PMID: 15213106
-
The connexin 30 A88V mutant reduces cochlear gap junction expression and confers long-term protection against hearing loss.J Cell Sci. 2019 Jan 16;132(2):jcs224097. doi: 10.1242/jcs.224097. J Cell Sci. 2019. PMID: 30559251
-
Conserved glycine at position 45 of major cochlear connexins constitutes a vital component of the Ca²⁺ sensor for gating of gap junction hemichannels.Biochem Biophys Res Commun. 2013 Jul 5;436(3):424-9. doi: 10.1016/j.bbrc.2013.05.118. Epub 2013 Jun 10. Biochem Biophys Res Commun. 2013. PMID: 23756814
-
Antibodies targeting extracellular domain of connexins for studies of hemichannels.Neuropharmacology. 2013 Dec;75:525-32. doi: 10.1016/j.neuropharm.2013.02.021. Epub 2013 Mar 13. Neuropharmacology. 2013. PMID: 23499293 Free PMC article. Review.
-
Harnessing the therapeutic potential of antibodies targeting connexin hemichannels.Biochim Biophys Acta Mol Basis Dis. 2021 Apr 1;1867(4):166047. doi: 10.1016/j.bbadis.2020.166047. Epub 2021 Jan 6. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 33418036 Review.
Cited by
-
Calcium Regulation of Connexin Hemichannels.Int J Mol Sci. 2024 Jun 15;25(12):6594. doi: 10.3390/ijms25126594. Int J Mol Sci. 2024. PMID: 38928300 Free PMC article. Review.
-
Connexin hemichannel inhibition improves skin pathology in Clouston syndrome mice.EBioMedicine. 2020 Jul;57:102856. doi: 10.1016/j.ebiom.2020.102856. Epub 2020 Jul 3. EBioMedicine. 2020. PMID: 32629388 Free PMC article. No abstract available.
-
Spatial and Temporal Localization of Connexins in Cells Using Confocal Microscopy.Methods Mol Biol. 2024;2801:57-74. doi: 10.1007/978-1-0716-3842-2_5. Methods Mol Biol. 2024. PMID: 38578413
-
Selection of Leptin Surrogates by a General Phenotypic Screening Method for Receptor Agonists.Biomolecules. 2024 Apr 9;14(4):457. doi: 10.3390/biom14040457. Biomolecules. 2024. PMID: 38672473 Free PMC article.
-
Biologic Therapies for the Management of Cutaneous Findings in Genodermatoses: A Review.Am J Clin Dermatol. 2022 Sep;23(5):673-688. doi: 10.1007/s40257-022-00700-4. Epub 2022 May 23. Am J Clin Dermatol. 2022. PMID: 35606649 Review.
References
-
- Garcia IE, Bosen F, Mujica P. From Hyperactive Connexin26 Hemichannels to Impairments in Epidermal Calcium Gradient and Permeability Barrier in the Keratitis-Ichthyosis-Deafness Syndrome. J Invest Dermatol. 2016;136(3):574–583. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

