Bioactivity of recombinant hFSH glycosylation variants in primary cultures of porcine granulosa cells
- PMID: 32553947
- PMCID: PMC7418035
- DOI: 10.1016/j.mce.2020.110911
Bioactivity of recombinant hFSH glycosylation variants in primary cultures of porcine granulosa cells
Abstract
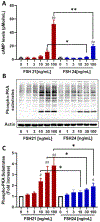
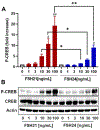
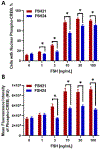
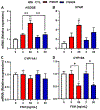
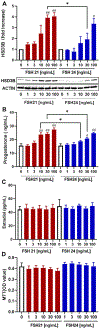
Previous studies have reported hypo-glycosylated FSH and fully-glycosylated FSH to be naturally occurring in humans, and these glycoforms exist in changing ratios over a woman's lifespan. The precise cellular and molecular effects of recombinant human FSH (hFSH) glycoforms, FSH21 and FSH24, have not been documented in primary granulosa cells. Herein, biological responses to FSH21 and FSH24 were compared in primary porcine granulosa cells. Hypo-glycosylated hFSH21 was significantly more effective than fully-glycosylated hFSH24 at stimulating cAMP accumulation and protein kinase A (PKA) activity, leading to the higher phosphorylation of CREB and β-Catenin. Compared to fully-glycosylated hFSH24, hypo-glycosylated hFSH21 also induced greater levels of transcripts for HSD3B, STAR and INHA, and higher progesterone production. Our results demonstrate that hypo-glycosylated hFSH21 exerts more robust activation of intracellular signals associated with steroidogenesis than fully-glycosylated hFSH24 in primary porcine granulosa cells, and furthers our understanding of the differing bioactivities of FSH glycoforms in the ovary.
Keywords: Cell signaling; Follicle; Ovary; Protein kinase A; Steroidogenesis; β-Catenin.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest None.
Figures




References
-
- Andreone L, Ambao V, Pellizzari EH, Loreti N, Cigorraga SB, Campo S. Role of FSH glycan structure in the regulation of Sertoli cell inhibin production. Reproduction. 2017; 154(5):711–721. - PubMed
-
- Bishop LA, Robertson DM, Cahir N, Schofield PR. Specific roles for the asparagine-linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. Mol Endocrinol. 1994; 8(6):722–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

