Downregulated brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein-1 inhibits osteogenesis of BMSCs through p53 in type 2 diabetes mellitus
- PMID: 32554484
- PMCID: PMC7358138
- DOI: 10.1242/bio.051482
Downregulated brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein-1 inhibits osteogenesis of BMSCs through p53 in type 2 diabetes mellitus
Retraction in
-
Retraction: Downregulated brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein-1 inhibits osteogenesis of BMSCs through p53 in type 2 diabetes mellitus.Biol Open. 2022 Jun 15;11(6):bio058581. doi: 10.1242/bio.058581. Epub 2022 Jun 28. Biol Open. 2022. PMID: 35762673 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern: Downregulated brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein-1 inhibits osteogenesis of BMSCs through p53 in type 2 diabetes mellitus.Biol Open. 2020 Oct 5;9(10):bio055525. doi: 10.1242/bio.055525. Biol Open. 2020. PMID: 33020142 Free PMC article. No abstract available.
Abstract
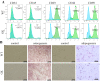
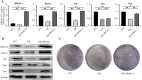
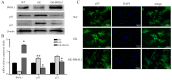
The bone marrow mesenchymal stem cells (BMSCs)-mediated abnormal bone metabolism can delay and impair the bone remodeling process in type 2 diabetes mellitus (T2DM). Our previous study demonstrated that the downregulation of brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL1), a circadian clock protein, inhibited the Wnt/β-catenin pathway via enhanced GSK-3β in diabetic BMSCs. In this article, we confirmed that the downregulated BMAL1 in T2DM played an inhibitory role in osteogenic differentiation of BMSCs. Upregulation of BMAL1 in the diabetic BMSCs significantly recovered the expression pattern of osteogenic marker genes and alkaline phosphatase (Alp) activity. We also observed an activation of the p53 signaling pathways, exhibited by increased p53 and p21 in diabetic BMSCs. Downregulation of p53 resulting from overexpression of BMAL1 was detected, and when we applied p53 gene silencing (shRNA) and the p53 inhibitor, pifithrin-α (PFT-α), the impaired osteogenic differentiation ability of diabetic BMSCs was greatly restored. However, there was no change in the level of expression of BMAL1. Taken together, our results first revealed that BMAL1 regulated osteogenesis of BMSCs through p53 in T2DM, providing a novel direction for further exploration of the mechanism underlying osteoporosis in diabetes.
Keywords: Bone marrow mesenchymal stem cells (BMSCs); Brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein-1 (BMAL1); Osteogenic differentiation; Type 2 diabetes mellitus (T2DM); p53.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Brown M. L., Yukata K., Farnsworth C. W., Chen D.-G., Awad H., Hilton M. J., O'Keefe R. J., Xing L., Mooney R. A. and Zuscik M. J. (2014). Delayed fracture healing and increased callus adiposity in a C57BL/6J murine model of obesity-associated type 2 diabetes mellitus. PLoS ONE 9, e99656 10.1371/journal.pone.0099656 - DOI - PMC - PubMed
-
- Degen R. M., Carbone A., Carballo C., Zong J., Chen T., Lebaschi A., Ying L., Deng X.-H. and Rodeo S. A. (2016). The effect of purified human bone marrow–derived mesenchymal stem cells on rotator cuff tendon healing in an athymic rat. Arthroscopy 32, 2435-2443. 10.1016/j.arthro.2016.04.019 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

