Development and function of smooth muscle cells is modulated by Hic1 in mouse testis
- PMID: 32554530
- PMCID: PMC7375483
- DOI: 10.1242/dev.185884
Development and function of smooth muscle cells is modulated by Hic1 in mouse testis
Abstract
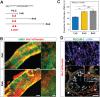
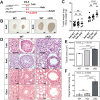
In mammalian testis, contractile peritubular myoid cells (PMCs) regulate the transport of sperm and luminal fluid, while secreting growth factors and extracellular matrix proteins to support the spermatogonial stem cell niche. However, little is known about the role of testicular smooth muscle cells during postnatal testicular development. Here we report age-dependent expression of hypermethylated in cancer 1 (Hic1; also known as ZBTB29) in testicular smooth muscle cells, including PMCs and vascular smooth muscle cells, in the mouse. Postnatal deletion of Hic1 in smooth muscle cells led to their increased proliferation and resulted in dilatation of seminiferous tubules, with increased numbers of PMCs. These seminiferous tubules contained fewer Sertoli cells and more spermatogonia, and fibronectin was not detected in their basement membrane. The expression levels of genes encoding smooth muscle contractile proteins, Acta2 and Cnn1, were downregulated in the smooth muscle cells lacking Hic1, and the seminiferous tubules appeared to have reduced contractility. These data imply a role for Hic1 in determining the size of seminiferous tubules by regulating postnatal smooth muscle cell proliferation, subsequently affecting spermatogenesis in adulthood.
Keywords: Fibronectin; Hic1; Mouse; Peritubular myoid cell; Testicular smooth muscle cells; Testis.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
-
- Agrimson K. S., Onken J., Mitchell D., Topping T. B., Chiarini-Garcia H., Hogarth C. A. and Griswold M. D. (2016). Characterizing the spermatogonial response to retinoic acid during the onset of spermatogenesis and following synchronization in the neonatal mouse testis. Biol. Reprod. 95, 81 10.1095/biolreprod.116.141770 - DOI - PMC - PubMed
-
- Akiyama S. K. (1996). Integrins in cell adhesion and signaling. Hum. Cell 9, 181-186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

