A Slow Short-Term Depression at Purkinje to Deep Cerebellar Nuclear Neuron Synapses Supports Gain-Control and Linear Encoding over Second-Long Time Windows
- PMID: 32554551
- PMCID: PMC7392510
- DOI: 10.1523/JNEUROSCI.2078-19.2020
A Slow Short-Term Depression at Purkinje to Deep Cerebellar Nuclear Neuron Synapses Supports Gain-Control and Linear Encoding over Second-Long Time Windows
Abstract
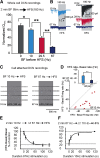
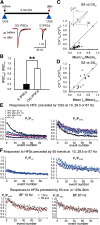
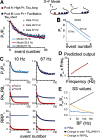
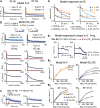
Modifications in the sensitivity of neural elements allow the brain to adapt its functions to varying demands. Frequency-dependent short-term synaptic depression (STD) provides a dynamic gain-control mechanism enabling adaptation to different background conditions alongside enhanced sensitivity to input-driven changes in activity. In contrast, synapses displaying frequency-invariant transmission can faithfully transfer ongoing presynaptic rates enabling linear processing, deemed critical for many functions. However, rigid frequency-invariant transmission may lead to runaway dynamics and low sensitivity to changes in rate. Here, I investigated the Purkinje cell to deep cerebellar nuclei neuron synapses (PC_DCNs), which display frequency invariance, and yet, PCs maintain background activity at disparate rates, even at rest. Using protracted PC_DCN activation (120 s) to mimic background activity in cerebellar slices from mature mice of both sexes, I identified a previously unrecognized, frequency-dependent, slow STD (S-STD), adapting IPSC amplitudes in tens of seconds to minutes. However, after changes in activation rates, over a behavior-relevant second-long time window, S-STD enabled scaled linear encoding of PC rates in synaptic charge transfer and DCN spiking activity. Combined electrophysiology, optogenetics, and statistical analysis suggested that S-STD mechanism is input-specific, involving decreased ready-to-release quanta, and distinct from faster short-term plasticity (f-STP). Accordingly, an S-STD component with a scaling effect (i.e., activity-dependent release sites inactivation), extending a model explaining PC_DCN release on shorter timescales using balanced f-STP, reproduced the experimental results. Thus, these results elucidates a novel slow gain-control mechanism able to support linear transfer of behavior-driven/learned PC rates concurrently with background activity adaptation, and furthermore, provides an alternative pathway to refine PC output.SIGNIFICANCE STATEMENT The brain can adapt to varying demands by dynamically changing the gain of its synapses; however, some tasks require ongoing linear transfer of presynaptic rates, seemingly incompatible with nonlinear gain adaptation. Here, I report a novel slow gain-control mechanism enabling scaled linear encoding of presynaptic rates over behavior-relevant time windows, and adaptation to background activity at the Purkinje to deep cerebellar nuclear neurons synapses (PC_DCNs). A previously unrecognized PC_DCNs slow and frequency-dependent short-term synaptic depression (S-STD) mediates this process. Experimental evidence and simulations suggested that scaled linear encoding emerges from the combination of S-STD slow dynamics and frequency-invariant transmission at faster timescales. These results demonstrate a mechanism reconciling rate code with background activity adaptation and suitable for flexibly tuning PCs output via background activity modulation.
Keywords: background activity; gain modulation; short-term memory; short-term plasticity; sustained activity; synaptic transmission.
Copyright © 2020 the authors.
Figures




References
-
- Belmeguenai A, Hosy E, Bengtsson F, Pedroarena CM, Piochon C, Teuling E, He Q, Ohtsuki G, De Jeu MT, Elgersma Y, De Zeeuw CI, Jörntell H, Hansel C (2010) Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci 30:13630–13643. 10.1523/JNEUROSCI.3226-10.2010 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
