The Adaptive designs CONSORT Extension (ACE) statement: a checklist with explanation and elaboration guideline for reporting randomised trials that use an adaptive design
- PMID: 32554564
- PMCID: PMC7298567
- DOI: 10.1136/bmj.m115
The Adaptive designs CONSORT Extension (ACE) statement: a checklist with explanation and elaboration guideline for reporting randomised trials that use an adaptive design
Abstract
Adaptive designs (ADs) allow pre-planned changes to an ongoing trial without compromising the validity of conclusions and it is essential to distinguish pre-planned from unplanned changes that may also occur. The reporting of ADs in randomised trials is inconsistent and needs improving. Incompletely reported AD randomised trials are difficult to reproduce and are hard to interpret and synthesise. This consequently hampers their ability to inform practice as well as future research and contributes to research waste. Better transparency and adequate reporting will enable the potential benefits of ADs to be realised.This extension to the Consolidated Standards Of Reporting Trials (CONSORT) 2010 statement was developed to enhance the reporting of randomised AD clinical trials. We developed an Adaptive designs CONSORT Extension (ACE) guideline through a two-stage Delphi process with input from multidisciplinary key stakeholders in clinical trials research in the public and private sectors from 21 countries, followed by a consensus meeting. Members of the CONSORT Group were involved during the development process.The paper presents the ACE checklists for AD randomised trial reports and abstracts, as well as an explanation with examples to aid the application of the guideline. The ACE checklist comprises seven new items, nine modified items, six unchanged items for which additional explanatory text clarifies further considerations for ADs, and 20 unchanged items not requiring further explanatory text. The ACE abstract checklist has one new item, one modified item, one unchanged item with additional explanatory text for ADs, and 15 unchanged items not requiring further explanatory text.The intention is to enhance transparency and improve reporting of AD randomised trials to improve the interpretability of their results and reproducibility of their methods, results and inference. We also hope indirectly to facilitate the much-needed knowledge transfer of innovative trial designs to maximise their potential benefits.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Figures
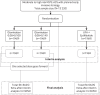

References
-
- CONSORT Group. Extensions of the CONSORT Statement. http://www.consort-statement.org/extensions.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
