Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial
- PMID: 32554566
- PMCID: PMC7301164
- DOI: 10.1136/bmj.m1822
Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial
Abstract
Objective: To evaluate the effects of a computerised decision support tool for comprehensive drug review in elderly people with polypharmacy.
Design: Pragmatic, multicentre, cluster randomised controlled trial.
Setting: 359 general practices in Austria, Germany, Italy, and the United Kingdom.
Participants: 3904 adults aged 75 years and older using eight or more drugs on a regular basis, recruited by their general practitioner.
Intervention: A newly developed electronic decision support tool comprising a comprehensive drug review to support general practitioners in deprescribing potentially inappropriate and non-evidence based drugs. Doctors were randomly allocated to either the electronic decision support tool or to provide treatment as usual.
Main outcome measures: The primary outcome was the composite of unplanned hospital admission or death by 24 months. The key secondary outcome was reduction in the number of drugs.
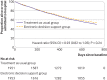
Results: 3904 adults were enrolled between January and October 2015. 181 practices and 1953 participants were assigned to electronic decision support (intervention group) and 178 practices and 1951 participants to treatment as usual (control group). The primary outcome (composite of unplanned hospital admission or death by 24 months) occurred in 871 (44.6%) participants in the intervention group and 944 (48.4%) in the control group. In an intention-to-treat analysis the odds ratio of the composite outcome was 0.88 (95% confidence interval 0.73 to 1.07; P=0.19, 997 of 1953 v 1055 of 1951). In an analysis restricted to participants attending practice according to protocol, a difference was found favouring the intervention (odds ratio 0.82, 95% confidence interval 0.68 to 0.98; 774 of 1682 v 873 of 1712, P=0.03). By 24 months the number of prescribed drugs had decreased in the intervention group compared with control group (uncontrolled mean change -0.42 v 0.06: adjusted mean difference -0.45, 95% confidence interval -0.63 to -0.26; P<0.001).
Conclusions: In intention-to-treat analysis, a computerised decision support tool for comprehensive drug review of elderly people with polypharmacy showed no conclusive effects on the composite of unplanned hospital admission or death by 24 months. Nonetheless, a reduction in drugs was achieved without detriment to patient outcomes.
Trial registration: Current Controlled Trials ISRCTN10137559.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: This study was funded by the seventh Framework Programme of the European Union, theme Health-2012-Innovation-1-2.2.2-2 (grant agreement No 305388-2). All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. IK is a salaried employee of Duodecim Medical Publications, a company that develops and sells the EBMeDS (evidence based medicine electronic decision support) service that was used as the technology platform of the intervention in the trial. All other authors declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Inappropriate medication use and polypharmacy in older people.BMJ. 2020 Jun 18;369:m2023. doi: 10.1136/bmj.m2023. BMJ. 2020. PMID: 32554409 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical