Combined DLL3-targeted bispecific antibody with PD-1 inhibition is efficient to suppress small cell lung cancer growth
- PMID: 32554616
- PMCID: PMC7304844
- DOI: 10.1136/jitc-2020-000785
Combined DLL3-targeted bispecific antibody with PD-1 inhibition is efficient to suppress small cell lung cancer growth
Abstract
Background: Small cell lung cancer (SCLC) accounts for 15% of lung cancers, and the primary treatment of this malignancy is chemotherapy and radiotherapy. Delta-like 3 (DLL3) is an attractive target for SCLC immunotherapy since its expression is highly restricted to SCLC with a neglectable appearance on normal adult tissues. In the current study, we aimed to explore the efficacy of DLL3-targeted SCLC immunotherapy via the engagement of T cell.
Methods: As a proof of concept, we constructed DLL3-targeted bispecific antibody and chimeric antigen receptor (CAR)-modified T cells. In vitro and in vivo tumor-suppression activity of these treatments alone or in combination with a Program Death-1 (PD-1) inhibitory antibody was evaluated.
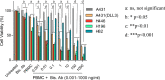
Results: In vitro studies showed that both DLL3 bispecific antibody and CAR-T efficiently killed DLL3-positive cancer cells, including the native SCLC cell lines H446, H196, H82, and the artificial A431 cells that were forcefully overexpressing DLL3. In vivo studies in xenograft mouse models demonstrated that both bispecific antibody and CAR-T suppressed the tumor growth, and combination therapy with PD-1 inhibitory antibody dramatically improved the efficacy of the DLL3 bispecific antibody, but not the CAR-T cells.
Conclusions: Our results demonstrated that DLL3-targeted bispecific antibody plus PD-1 inhibition was effective in controlling SCLC growth.
Keywords: antibodies; biomarkers; immunotherapy; lung neoplasms; neoplasm; tumor.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




Similar articles
-
TREM1/DAP12 based novel multiple chain CAR-T cells targeting DLL3 show robust anti-tumour efficacy for small cell lung cancer.Immunology. 2024 Jul;172(3):362-374. doi: 10.1111/imm.13776. Epub 2024 Mar 12. Immunology. 2024. PMID: 38469682
-
A Bispecific DLL3/CD3 IgG-Like T-Cell Engaging Antibody Induces Antitumor Responses in Small Cell Lung Cancer.Clin Cancer Res. 2020 Oct 1;26(19):5258-5268. doi: 10.1158/1078-0432.CCR-20-0926. Epub 2020 Jun 18. Clin Cancer Res. 2020. PMID: 32554516
-
AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer.Clin Cancer Res. 2021 Mar 1;27(5):1526-1537. doi: 10.1158/1078-0432.CCR-20-2845. Epub 2020 Nov 17. Clin Cancer Res. 2021. PMID: 33203642
-
Harnessing delta-like ligand 3: bridging biomarker discovery to next-generation immunotherapies in refractory small cell lung cancer.Front Immunol. 2025 May 27;16:1592291. doi: 10.3389/fimmu.2025.1592291. eCollection 2025. Front Immunol. 2025. PMID: 40496850 Free PMC article. Review.
-
DLL3: an emerging target in small cell lung cancer.J Hematol Oncol. 2019 Jun 18;12(1):61. doi: 10.1186/s13045-019-0745-2. J Hematol Oncol. 2019. PMID: 31215500 Free PMC article. Review.
Cited by
-
Strengthening of antitumor effects in breast cancer from a novel B7-H4- and CD3-targeting bispecific antibody by an oncolytic virus.Ann Transl Med. 2022 Jul;10(14):805. doi: 10.21037/atm-22-3423. Ann Transl Med. 2022. PMID: 35965796 Free PMC article.
-
DLL3 Is a Prognostic and Potentially Predictive Biomarker for Immunotherapy Linked to PD/PD-L Axis and NOTCH1 in Pancreatic Cancer.Biomedicines. 2023 Oct 17;11(10):2812. doi: 10.3390/biomedicines11102812. Biomedicines. 2023. PMID: 37893184 Free PMC article.
-
Potent molecular-targeted therapies for gastro-entero-pancreatic neuroendocrine carcinoma.Cancer Metastasis Rev. 2023 Sep;42(3):1021-1054. doi: 10.1007/s10555-023-10121-2. Epub 2023 Jul 8. Cancer Metastasis Rev. 2023. PMID: 37422534 Free PMC article. Review.
-
Promising therapy for neuroendocrine prostate cancer: current status and future directions.Ther Adv Med Oncol. 2024 Aug 8;16:17588359241269676. doi: 10.1177/17588359241269676. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39131727 Free PMC article. Review.
-
CAR T cells in solid tumors: challenges and opportunities.Stem Cell Res Ther. 2021 Jan 25;12(1):81. doi: 10.1186/s13287-020-02128-1. Stem Cell Res Ther. 2021. PMID: 33494834 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
