Immune-related (IR)-pneumonitis during the COVID-19 pandemic: multidisciplinary recommendations for diagnosis and management
- PMID: 32554619
- PMCID: PMC7316105
- DOI: 10.1136/jitc-2020-000984
Immune-related (IR)-pneumonitis during the COVID-19 pandemic: multidisciplinary recommendations for diagnosis and management
Abstract
Immune-related (IR)-pneumonitis is a rare and potentially fatal toxicity of anti-PD(L)1 immunotherapy. Expert guidelines for the diagnosis and management of IR-pneumonitis include multidisciplinary input from medical oncology, pulmonary medicine, infectious disease, and radiology specialists. Severe acute respiratory syndrome coronavirus 2 is a recently recognized respiratory virus that is responsible for causing the COVID-19 global pandemic. Symptoms and imaging findings from IR-pneumonitis and COVID-19 pneumonia can be similar, and early COVID-19 viral testing may yield false negative results, complicating the diagnosis and management of both entities. Herein, we present a set of multidisciplinary consensus recommendations for the diagnosis and management of IR-pneumonitis in the setting of COVID-19 including: (1) isolation procedures, (2) recommended imaging and interpretation, (3) adaptations to invasive testing, (4) adaptations to the management of IR-pneumonitis, (5) immunosuppression for steroid-refractory IR-pneumonitis, and (6) management of suspected concurrent IR-pneumonitis and COVID-19 infection. There is an emerging need for the adaptation of expert guidelines for IR-pneumonitis in the setting of the global COVID-19 pandemic. We propose a multidisciplinary consensus on this topic, in this position paper.
Keywords: autoimmunity; guidelines as topic; immunotherapy.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JN: research funding: Merck, AstraZeneca; consulting/advisory board: Bristol-Myers Squibb, AstraZeneca, Roche/Genentech; honoraria: Bristol-Myers Squibb, Merck, AstraZeneca. DBJ: advisory boards/consulting: Array Biopharma, BMS, Jansen, Merck, Novartis; research funding: BMS, Incyte. JRB: advisory boards/consulting: Amgen, BMS, Genentech/Roche, Eli Lilly, GlaxoSmithKline, Merck, Sanofi; research funding: AstraZeneca, BMS, Genentech/Roche, Merck, RAPT Therapeutics Inc., Revolution Medicines; data and safety monitoring board/committees: GlaxoSmithKline, Sanofi. MN: advisory boards/consulting: Daiichi Sankyo, AstraZeneca; research funding: Merck, Canon Medical Systems, AstraZeneca, Daiichi Sankyo; honorarium: Roche. PMF: advisory boards/consulting: Abbvie, AstraZeneca, BMS; research funding: AstraZeneca, BMS, Kyowa, Novartis, Corvus.
Figures
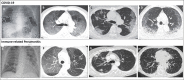
Similar articles
-
Immunotherapy is a preferred option for oral cancer patients during COVID-19 pandemic?Oral Oncol. 2020 Aug;107:104860. doi: 10.1016/j.oraloncology.2020.104860. Epub 2020 Jun 15. Oral Oncol. 2020. PMID: 32571643 Free PMC article. No abstract available.
-
Impact of COVID-19 outbreak on cancer immunotherapy in Italy: a survey of young oncologists.J Immunother Cancer. 2020 Oct;8(2):e001154. doi: 10.1136/jitc-2020-001154. J Immunother Cancer. 2020. PMID: 33060148 Free PMC article.
-
Impact of COVID-19 on patients with rheumatic complications of cancer immunotherapy: results of a registry survey.J Immunother Cancer. 2020 Oct;8(2):e001550. doi: 10.1136/jitc-2020-001550. J Immunother Cancer. 2020. PMID: 33067320 Free PMC article.
-
A Practical Approach to the Management of Cancer Patients During the Novel Coronavirus Disease 2019 (COVID-19) Pandemic: An International Collaborative Group.Oncologist. 2020 Jun;25(6):e936-e945. doi: 10.1634/theoncologist.2020-0213. Epub 2020 Apr 27. Oncologist. 2020. PMID: 32243668 Free PMC article. Review.
-
Recommendations on Management of Locally Advanced Rectal Cancer During the COVID-19 Pandemic: an Iranian Consensus.J Gastrointest Cancer. 2020 Sep;51(3):800-804. doi: 10.1007/s12029-020-00454-4. J Gastrointest Cancer. 2020. PMID: 32656628 Free PMC article. Review.
Cited by
-
Optimizing inpatient care for lung cancer patients with immune checkpoint inhibitor-related pneumonitis using a clinical care pathway algorithm.Support Care Cancer. 2024 Sep 16;32(10):661. doi: 10.1007/s00520-024-08867-8. Support Care Cancer. 2024. PMID: 39283351
-
Screening for COVID-19 in Symptomatic Cancer Patients in a Cancer Hospital.Cancer Cell. 2020 Nov 9;38(5):609-610. doi: 10.1016/j.ccell.2020.09.017. Epub 2020 Oct 2. Cancer Cell. 2020. PMID: 33064994 Free PMC article. No abstract available.
-
An Overview of Thermal Infrared Imaging-Based Screenings during Pandemic Emergencies.Int J Environ Res Public Health. 2021 Mar 22;18(6):3286. doi: 10.3390/ijerph18063286. Int J Environ Res Public Health. 2021. PMID: 33810086 Free PMC article. Review.
-
Immunotherapy for Stage III NSCLC: Durvalumab and Beyond.Lung Cancer (Auckl). 2021 Nov 2;12:123-131. doi: 10.2147/LCTT.S305466. eCollection 2021. Lung Cancer (Auckl). 2021. PMID: 34754256 Free PMC article. Review.
-
Differential diagnosis of COVID-19 at the chest computed tomography scan: A review with special focus on cancer patients.World J Radiol. 2021 Aug 28;13(8):243-257. doi: 10.4329/wjr.v13.i8.243. World J Radiol. 2021. PMID: 34567434 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
