Immune-related (IR)-pneumonitis during the COVID-19 pandemic: multidisciplinary recommendations for diagnosis and management
- PMID: 32554619
- PMCID: PMC7316105
- DOI: 10.1136/jitc-2020-000984
Immune-related (IR)-pneumonitis during the COVID-19 pandemic: multidisciplinary recommendations for diagnosis and management
Abstract
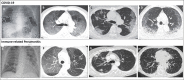
Immune-related (IR)-pneumonitis is a rare and potentially fatal toxicity of anti-PD(L)1 immunotherapy. Expert guidelines for the diagnosis and management of IR-pneumonitis include multidisciplinary input from medical oncology, pulmonary medicine, infectious disease, and radiology specialists. Severe acute respiratory syndrome coronavirus 2 is a recently recognized respiratory virus that is responsible for causing the COVID-19 global pandemic. Symptoms and imaging findings from IR-pneumonitis and COVID-19 pneumonia can be similar, and early COVID-19 viral testing may yield false negative results, complicating the diagnosis and management of both entities. Herein, we present a set of multidisciplinary consensus recommendations for the diagnosis and management of IR-pneumonitis in the setting of COVID-19 including: (1) isolation procedures, (2) recommended imaging and interpretation, (3) adaptations to invasive testing, (4) adaptations to the management of IR-pneumonitis, (5) immunosuppression for steroid-refractory IR-pneumonitis, and (6) management of suspected concurrent IR-pneumonitis and COVID-19 infection. There is an emerging need for the adaptation of expert guidelines for IR-pneumonitis in the setting of the global COVID-19 pandemic. We propose a multidisciplinary consensus on this topic, in this position paper.
Keywords: autoimmunity; guidelines as topic; immunotherapy.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JN: research funding: Merck, AstraZeneca; consulting/advisory board: Bristol-Myers Squibb, AstraZeneca, Roche/Genentech; honoraria: Bristol-Myers Squibb, Merck, AstraZeneca. DBJ: advisory boards/consulting: Array Biopharma, BMS, Jansen, Merck, Novartis; research funding: BMS, Incyte. JRB: advisory boards/consulting: Amgen, BMS, Genentech/Roche, Eli Lilly, GlaxoSmithKline, Merck, Sanofi; research funding: AstraZeneca, BMS, Genentech/Roche, Merck, RAPT Therapeutics Inc., Revolution Medicines; data and safety monitoring board/committees: GlaxoSmithKline, Sanofi. MN: advisory boards/consulting: Daiichi Sankyo, AstraZeneca; research funding: Merck, Canon Medical Systems, AstraZeneca, Daiichi Sankyo; honorarium: Roche. PMF: advisory boards/consulting: Abbvie, AstraZeneca, BMS; research funding: AstraZeneca, BMS, Kyowa, Novartis, Corvus.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
