The Functional Characterization of TcMyoF Implicates a Family of Cytostome-Cytopharynx Targeted Myosins as Integral to the Endocytic Machinery of Trypanosoma cruzi
- PMID: 32554712
- PMCID: PMC7300353
- DOI: 10.1128/mSphere.00313-20
The Functional Characterization of TcMyoF Implicates a Family of Cytostome-Cytopharynx Targeted Myosins as Integral to the Endocytic Machinery of Trypanosoma cruzi
Abstract
Of the pathogenic trypanosomatids, Trypanosoma cruzi alone retains an ancient feeding apparatus known as the cyto
Keywords: SPC; Trypanosoma cruzi; cytopharynx; cytostome; endocytosis; myosin; reservosome.
Copyright © 2020 Chasen et al.
Figures

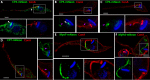
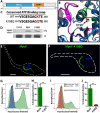

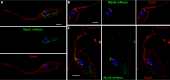

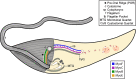
References
-
- Weekly Epidemiological Record. 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90:33–43. - PubMed
-
- Mejía-Jaramillo AM, Fernández GJ, Montilla M, Nicholls RS, Triana-Chávez O. 2012. Trypanosoma cruzi strains resistant to benznidazole occurring in Colombia. Biomedica 32:196–205. (In Spanish.) - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
