Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder
- PMID: 32554760
- PMCID: PMC7455322
- DOI: 10.1212/WNL.0000000000009758
Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder
Abstract
Objective: Myelin oligodendrocyte glycoprotein-immunoglobulin G (MOG-IgG) associated disorder (MOGAD) often manifests with recurrent CNS demyelinating attacks. The optimal treatment for reducing relapses is unknown. To help determine the efficacy of long-term immunotherapy in preventing relapse in patients with MOGAD, we conducted a multicenter retrospective study to determine the rate of relapses on various treatments.
Methods: We determined the frequency of relapses in patients receiving various forms of long-term immunotherapy for MOGAD. Inclusion criteria were history of ≥1 CNS demyelinating attacks, MOG-IgG seropositivity, and immunotherapy for ≥6 months. Patients were reviewed for CNS demyelinating attacks before and during long-term immunotherapy.
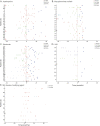
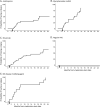
Results: Seventy patients were included. The median age at initial CNS demyelinating attack was 29 years (range 3-61 years; 33% <18 years), and 59% were female. The median annualized relapse rate (ARR) before treatment was 1.6. On maintenance immunotherapy, the proportion of patients with relapse was as follows: mycophenolate mofetil 74% (14 of 19; ARR 0.67), rituximab 61% (22 of 36; ARR 0.59), azathioprine 59% (13 of 22; ARR 0.2), and IV immunoglobulin (IVIG) 20% (2 of 10; ARR 0). The overall median ARR on these 4 treatments was 0.3. All 9 patients treated with multiple sclerosis (MS) disease-modifying agents had a breakthrough relapse on treatment (ARR 1.5).
Conclusion: This large retrospective multicenter study of patients with MOGAD suggests that maintenance immunotherapy reduces recurrent CNS demyelinating attacks, with the lowest ARR being associated with maintenance IVIG therapy. Traditional MS disease-modifying agents appear to be ineffective. Prospective randomized controlled studies are required to validate these conclusions.
© 2020 American Academy of Neurology.
Figures
Comment in
-
We need to talk about MOG.Neurology. 2020 Jul 14;95(2):55-56. doi: 10.1212/WNL.0000000000009768. Epub 2020 Jun 17. Neurology. 2020. PMID: 32554761 No abstract available.
References
-
- Jurynczyk M, Messina S, Woodhall MR, et al. . Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 2017;140:3128–3138. - PubMed
-
- Cobo-Calvo A, Ruiz A, Maillart E, et al. . Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology 2018;90:e1858–e1869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
