Sources of off-target effects of vagus nerve stimulation using the helical clinical lead in domestic pigs
- PMID: 32554888
- PMCID: PMC7717671
- DOI: 10.1088/1741-2552/ab9db8
Sources of off-target effects of vagus nerve stimulation using the helical clinical lead in domestic pigs
Abstract
Objective: Clinical data suggest that efficacious vagus nerve stimulation (VNS) is limited by side effects such as cough and dyspnea that have stimulation thresholds lower than those for therapeutic outcomes. VNS side effects are putatively caused by activation of nearby muscles within the neck, via direct muscle activation or activation of nerve fibers innervating those muscles. Our goal was to determine the thresholds at which various VNS-evoked effects occur in the domestic pig—an animal model with vagus anatomy similar to human—using the bipolar helical lead deployed clinically.
Approach: Intrafascicular electrodes were placed within the vagus nerve to record electroneurographic (ENG) responses, and needle electrodes were placed in the vagal-innervated neck muscles to record electromyographic (EMG) responses.
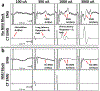
Main results: Contraction of the cricoarytenoid muscle occurred at low amplitudes (~0.3 mA) and resulted from activation of motor nerve fibers in the cervical vagus trunk within the electrode cuff which bifurcate into the recurrent laryngeal branch of the vagus. At higher amplitudes (~1.4 mA), contraction of the cricoarytenoid and cricothyroid muscles was generated by current leakage outside the cuff to activate motor nerve fibers running within the nearby superior laryngeal branch of the vagus. Activation of these muscles generated artifacts in the ENG recordings that may be mistaken for compound action potentials representing slowly conducting Aδ-, B-, and C-fibers.
Significance: Our data resolve conflicting reports of the stimulation amplitudes required for C-fiber activation in large animal studies (>10 mA) and human studies (<250 μA). After removing muscle-generated artifacts, ENG signals with post-stimulus latencies consistent with Aδ- and B-fibers occurred in only a small subset of animals, and these signals had similar thresholds to those that caused bradycardia. By identifying specific neuroanatomical pathways that cause off-target effects and characterizing the stimulation dose-response curves for on- and off-target effects, we hope to guide interpretation and optimization of clinical VNS.
Conflict of interest statement
Conflict of interest
JW and KAL are scientific board members and have stock interests in NeuroOne Medical Inc., a company developing next generation epilepsy monitoring devices. JW also has an equity interest in NeuroNexus technology Inc., a company that supplies electrophysiology equipment and multichannel probes to the neuroscience research community. KAL is also paid member of the scientific advisory board of Cala Health, Blackfynn, Abbott and Battelle. KAL also is a paid consultant for Galvani and Boston Scientific. KAL is a consultant to and co-founder of Neuronoff Inc. None of these associations are directly relevant to the work presented in this manuscript.
Figures





References
-
- Altschuler SM, Bao X, Bieger D, Hopkins DA and Miselis RR 1989. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts J. Comp. Neurol 283 248–68 - PubMed
-
- Anholt TA, Ayal S and Goldberg JA 2011. Recruitment and blocking properties of the CardioFit stimulation lead J. Neural. Eng 8 034004. - PubMed
-
- Aristovich K, Model-based geometrical optimisation and in vivo validation of a spatially selective multielectrode cuff array for vagus nerve neuromodulation (arXiv:1903.12459) 2019. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
