The complement system in COVID-19: friend and foe?
- PMID: 32554923
- PMCID: PMC7455060
- DOI: 10.1172/jci.insight.140711
The complement system in COVID-19: friend and foe?
Abstract
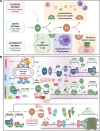
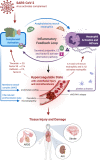
Coronavirus disease 2019 (COVID-19), the disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has resulted in a global pandemic and a disruptive health crisis. COVID-19-related morbidity and mortality have been attributed to an exaggerated immune response. The role of complement activation and its contribution to illness severity is being increasingly recognized. Here, we summarize current knowledge about the interaction of coronaviruses with the complement system. We posit that (a) coronaviruses activate multiple complement pathways; (b) severe COVID-19 clinical features often resemble complementopathies; (c) the combined effects of complement activation, dysregulated neutrophilia, endothelial injury, and hypercoagulability appear to be intertwined to drive the severe features of COVID-19; (d) a subset of patients with COVID-19 may have a genetic predisposition associated with complement dysregulation; and (e) these observations create a basis for clinical trials of complement inhibitors in life-threatening illness.
Trial registration: ClinicalTrials.gov NCT04369469.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

