Decreased Anti-Müllerian hormone and Anti-Müllerian hormone receptor type 2 in hypothalami of old Japanese Black cows
- PMID: 32554955
- PMCID: PMC7468072
- DOI: 10.1292/jvms.20-0159
Decreased Anti-Müllerian hormone and Anti-Müllerian hormone receptor type 2 in hypothalami of old Japanese Black cows
Abstract
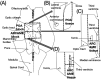
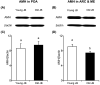
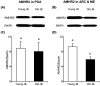
Cow fertility decreases with age, but the hypothalamic pathomechanisms are not understood. Anti-Müllerian hormone (AMH) stimulates gonadotropin-releasing hormone (GnRH) neurons via AMH receptor type 2 (AMHR2), and most GnRH neurons in the preoptic area (POA), arcuate nucleus (ARC), and median eminence (ME) express AMH and AMHR2. Therefore, we hypothesized that both protein amounts would differ in the anterior hypothalamus (containing the POA) and posterior hypothalamus (containing the ARC and ME) between young post-pubertal heifers and old cows. Western blot analysis showed lower (P<0.05) expressions of AMH and AMHR2 in the posterior hypothalamus, but not in the anterior hypothalamus, of old Japanese Black cows compared to young heifers. Therefore, AMH and AMHR2 were decreased in the posterior hypothalami of old cows.
Keywords: Müllerian inhibiting substance; female reproductive senescence; gonadotropin-releasing hormone neuron; preoptic area; ruminant.
Figures
References
-
- Bond B. C., Virley D. J., Cairns N. J., Hunter A. J., Moore G. B., Moss S. J., Mudge A. W., Walsh F. S., Jazin E., Preece P.2002. The quantification of gene expression in an animal model of brain ischaemia using TaqMan real-time RT-PCR. Brain Res. Mol. Brain Res. 106: 101–116. doi: 10.1016/S0169-328X(02)00417-5 - DOI - PubMed
-
- Cimino I., Casoni F., Liu X., Messina A., Parkash J., Jamin S. P., Catteau-Jonard S., Collier F., Baroncini M., Dewailly D., Pigny P., Prescott M., Campbell R., Herbison A. E., Prevot V., Giacobini P.2016. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat. Commun. 7: 10055. doi: 10.1038/ncomms10055 - DOI - PMC - PubMed
-
- Hassaneen A., Naniwa Y., Suetomi Y., Matsuyama S., Kimura K., Ieda N., Inoue N., Uenoyama Y., Tsukamura H., Maeda K. I., Matsuda F., Ohkura S.2016. Immunohistochemical characterization of the arcuate kisspeptin/neurokinin B/dynorphin (KNDy) and preoptic kisspeptin neuronal populations in the hypothalamus during the estrous cycle in heifers. J. Reprod. Dev. 62: 471–477. doi: 10.1262/jrd.2016-075 - DOI - PMC - PubMed
-
- Kamomae H.2012. Reproductive disturbance. pp. 283–340. In: Veterinary Theriogenology (Nakao, T., Tsumagari, S. and Katagiri, S. eds.), Buneidou Press, Tokyo (in Japanese).

