The Limited Sensitivity of Chest Computed Tomography Relative to Reverse Transcription Polymerase Chain Reaction for Severe Acute Respiratory Syndrome Coronavirus-2 Infection: A Systematic Review on COVID-19 Diagnostics
- PMID: 32554983
- PMCID: PMC7314354
- DOI: 10.1097/RLI.0000000000000700
The Limited Sensitivity of Chest Computed Tomography Relative to Reverse Transcription Polymerase Chain Reaction for Severe Acute Respiratory Syndrome Coronavirus-2 Infection: A Systematic Review on COVID-19 Diagnostics
Abstract
Objectives: Several studies suggest the sensitivity of chest computed tomography (CT) is far greater than that of reverse transcription polymerase chain reaction (RT-PCR) in diagnosing COVID-19 patients, and therefore, CT should be included as a primary diagnostic tool. This systematic review aims to stratify studies as high or low risk of bias to determine the true sensitivity of CT for severe acute respiratory syndrome coronavirus-2 infection according to the unbiased (low risk) studies, a topic of particular importance given the insufficient quantity of RT-PCR kits in many countries. We focus on sensitivity as that is the chief advantage perceived of CT.
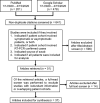
Materials and methods: This systematic review involved searching the PubMed and Google Scholar databases for articles conducted and published between January 1 and April 15, 2020. The quality assessment tool QUADAS-2 was used to stratify studies according to their risk of bias, and exclusion criteria included not providing the information deemed relevant for such a stratification, such as not indicating if the patients were symptomatic or asymptomatic, or identifying the source of the specimen for the reference standard, RT-PCR (eg, nasal, oropharyngeal, etc). Sensitivity values were then extracted, and random effects meta-analyses were performed.
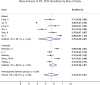
Results: Of 641 search results, 37 studies (n = 9610 patients) were included in the analysis. The mean sensitivity of RT-PCR for COVID-19 reported by the biased studies was 70% (n = 5409/7 studies; 95% confidence interval [CI], 43-97; I = 99.1%), compared with 78% by unbiased studies (n = 534/4 studies; 95% CI, 69-87, I = 89.9%). For chest CT, the mean sensitivity reported by biased studies was 94% (n = 3371 patients/24 studies; 95% CI, 92-96; I = 93.1%), compared with 75% by unbiased studies (n = 957/10 studies; 95% CI, 67-83; I = 89.5%).
Conclusions: The difference between the sensitivities of CT and RT-PCR for severe acute respiratory syndrome coronavirus-2 infection is lower than previously thought, as after stratifying the studies, the true sensitivity for CT based on the unbiased studies is limited.
Conflict of interest statement
Conflicts of interest and sources of funding: none declared.
Figures

References
-
- World Map. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/world-map.html. Accessed April 19, 2020.
-
- Centers for Disease Control and Prevention. Cases in U.S. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed April 19, 2020.
-
- Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) [published online ahead of print]. Clin Chem Lab Med. - PubMed

