Organic NIR-II molecule with long blood half-life for in vivo dynamic vascular imaging
- PMID: 32555157
- PMCID: PMC7303218
- DOI: 10.1038/s41467-020-16924-z
Organic NIR-II molecule with long blood half-life for in vivo dynamic vascular imaging
Abstract
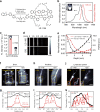
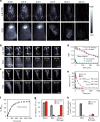
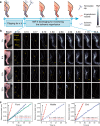
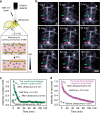
Real-time monitoring of vessel dysfunction is of great significance in preclinical research. Optical bioimaging in the second near-infrared (NIR-II) window provides advantages including high resolution and fast feedback. However, the reported molecular dyes are hampered by limited blood circulation time (~ 5-60 min) and short absorption and emission wavelength, which impede the accurate long-term monitoring. Here, we report a NIR-II molecule (LZ-1105) with absorption and emission beyond 1000 nm. Thanks to the long blood circulation time (half-life of 3.2 h), the fluorophore is used for continuous real-time monitoring of dynamic vascular processes, including ischemic reperfusion in hindlimbs, thrombolysis in carotid artery and opening and recovery of the blood brain barrier (BBB). LZ-1105 provides an approach for researchers to assess vessel dysfunction due to the long excitation and emission wavelength and long-term blood circulation properties.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. - PubMed
-
- Pasterkamp G, den Ruijter HM, Libby P. Temporal shifts in clinical presentation and underlying mechanisms of atherosclerotic disease. Nat. Rev. Cardiol. 2017;14:21–29. - PubMed
-
- Tsukasaki Y, et al. A short-wavelength infrared emitting multimodal probe for non-invasive visualization of phagocyte cell migration in living mice. Chem. Commun. 2014;50:14356–14359. - PubMed
-
- Hong G, Antaris AL, Dai H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017;1:0010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

