NORAD orchestrates endometrial cancer progression by sequestering FUBP1 nuclear localization to promote cell apoptosis
- PMID: 32555178
- PMCID: PMC7303217
- DOI: 10.1038/s41419-020-2674-y
NORAD orchestrates endometrial cancer progression by sequestering FUBP1 nuclear localization to promote cell apoptosis
Abstract
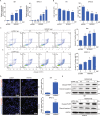
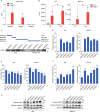
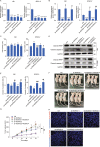
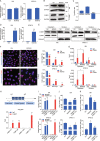
Long noncoding RNAs (lncRNAs) are emerging as critical regulators in tumor initiation and progression. However, the biological mechanisms and potential clinical application of lncRNA NORAD in endometrial cancer (EC) remain unknown. Herein, we identified NORAD underwent promoter hypermethylation-associated downregulation in EC. Epigenetic inactivation of NORAD was correlated with EC progression (FIGO stage) and poor outcome. Overexpression of NORAD significantly inhibited cell growth and promoted apoptosis in EC cells. Mechanistic studies revealed that multiple regions of NORAD served as a platform for binding with the central domain of anti-apoptotic factor FUBP1. Our findings further indicated that the NORAD/FUBP1 interaction attenuated FUBP1 nuclear localization and thus impaired the occupancies of FUBP1 on its target pro-apoptotic gene promoters, resulting in apoptosis induction in EC. Moreover, knockdown of NORAD promoted tumor growth in the xenograft mice model. While, introduction of NORAD-4 fragment, which bound with FUBP1, successfully reversed tumor growth and apoptosis inhibition mediated by NORAD knockdown in vivo. Our findings provide mechanistic insight into the critical roles of NORAD as a tumor suppressor in EC progression. NORAD could possibly serve as a novel prognostic biomarker and provide the rationale for EC therapy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
STAT1-induced upregulation of lncRNA LINC01123 predicts poor prognosis and promotes the progression of endometrial cancer through miR-516b/KIF4A.Cell Cycle. 2020 Jun;19(12):1502-1516. doi: 10.1080/15384101.2020.1757936. Epub 2020 May 13. Cell Cycle. 2020. Retraction in: Cell Cycle. 2023 May;22(9):1158. doi: 10.1080/15384101.2023.2188826. PMID: 32401659 Free PMC article. Retracted.
-
Long non-coding RNA NORAD upregulate SIP1 expression to promote cell proliferation and invasion in cervical cancer.Biomed Pharmacother. 2018 Oct;106:1454-1460. doi: 10.1016/j.biopha.2018.07.101. Epub 2018 Jul 24. Biomed Pharmacother. 2018. PMID: 30119219
-
Long non-coding RNA DLX6-AS1 mediates proliferation, invasion and apoptosis of endometrial cancer cells by recruiting p300/E2F1 in DLX6 promoter region.J Cell Mol Med. 2020 Nov;24(21):12572-12584. doi: 10.1111/jcmm.15810. Epub 2020 Sep 20. J Cell Mol Med. 2020. PMID: 32951317 Free PMC article.
-
NORAD, a critical long non-coding RNA in human cancers.Life Sci. 2021 Jan 1;264:118665. doi: 10.1016/j.lfs.2020.118665. Epub 2020 Oct 27. Life Sci. 2021. PMID: 33127516 Review.
-
The master regulator FUBP1: its emerging role in normal cell function and malignant development.Cell Mol Life Sci. 2019 Jan;76(2):259-281. doi: 10.1007/s00018-018-2933-6. Epub 2018 Oct 20. Cell Mol Life Sci. 2019. PMID: 30343319 Free PMC article. Review.
Cited by
-
LncRNAs-associated to genomic instability: A barrier to cancer therapy effectiveness.Front Genet. 2022 Nov 21;13:984329. doi: 10.3389/fgene.2022.984329. eCollection 2022. Front Genet. 2022. PMID: 36479250 Free PMC article.
-
LncRNAs signatures in gynecological cancers: ovarian and endometrial cancers.Clin Transl Oncol. 2025 Aug 11. doi: 10.1007/s12094-025-04020-x. Online ahead of print. Clin Transl Oncol. 2025. PMID: 40790288 Review.
-
The Role of m6A RNA Methylation-Related lncRNAs in the Prognosis and Tumor Immune Microenvironment of Papillary Thyroid Carcinoma.Front Cell Dev Biol. 2022 Jan 3;9:719820. doi: 10.3389/fcell.2021.719820. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35047491 Free PMC article.
-
TNPO1-Mediated Nuclear Import of FUBP1 Contributes to Tumor Immune Evasion by Increasing NRP1 Expression in Cervical Cancer.J Immunol Res. 2021 Apr 24;2021:9994004. doi: 10.1155/2021/9994004. eCollection 2021. J Immunol Res. 2021. PMID: 33987449 Free PMC article.
-
SIRT1-mediated deacetylation of FOXO3 enhances mitophagy and drives hormone resistance in endometrial cancer.Mol Med. 2024 Sep 12;30(1):147. doi: 10.1186/s10020-024-00915-7. Mol Med. 2024. PMID: 39266959 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. - PubMed
-
- Brooks RA, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J. Clin. 2019;69:258–279. - PubMed
-
- Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

