Acyl homoserine lactone-mediated quorum sensing in the oral cavity: a paradigm revisited
- PMID: 32555242
- PMCID: PMC7300016
- DOI: 10.1038/s41598-020-66704-4
Acyl homoserine lactone-mediated quorum sensing in the oral cavity: a paradigm revisited
Abstract
Acyl homoserine lactones (AHLs), the quorum sensing (QS) signals produced by Gram-negative bacteria, are currently considered to play a minor role in the development of oral biofilm since their production by oral pathogens has not been ascertained thus far. However, we report the presence of AHLs in different oral samples and their production by the oral pathogen Porphyromonas gingivalis. The importance of AHLs is further supported by a very high prevalence of AHL-degradation capability, up to 60%, among bacteria isolated from dental plaque and saliva samples. Furthermore, the wide-spectrum AHL-lactonase Aii20J significantly inhibited oral biofilm formation in different in vitro biofilm models and caused important changes in bacterial composition. Besides, the inhibitory effect of Aii20J on a mixed biofilm of 6 oral pathogens was verified using confocal microscopy. Much more research is needed in order to be able to associate specific AHLs with oral pathologies and to individuate the key actors in AHL-mediated QS processes in dental plaque formation. However, these results indicate a higher relevance of the AHLs in the oral cavity than generally accepted thus far and suggest the potential use of inhibitory strategies against these signals for the prevention and treatment of oral diseases.
Conflict of interest statement
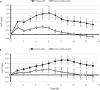
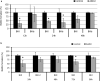
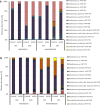
The anti-biofilm activity of
Figures





Similar articles
-
Silent signals: how N-acyl homoserine lactones drive oral microbial behaviour and health outcomes.Front Oral Health. 2024 Dec 5;5:1484005. doi: 10.3389/froh.2024.1484005. eCollection 2024. Front Oral Health. 2024. PMID: 39703871 Free PMC article. Review.
-
The quorum quenching enzyme Aii20J modifies in vitro periodontal biofilm formation.Front Cell Infect Microbiol. 2023 Feb 2;13:1118630. doi: 10.3389/fcimb.2023.1118630. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36816581 Free PMC article.
-
Short-Chain N-Acylhomoserine Lactone Quorum-Sensing Molecules Promote Periodontal Pathogens in In Vitro Oral Biofilms.Appl Environ Microbiol. 2020 Jan 21;86(3):e01941-19. doi: 10.1128/AEM.01941-19. Print 2020 Jan 21. Appl Environ Microbiol. 2020. PMID: 31757829 Free PMC article.
-
Role of N-acyl homoserine lactone (AHL)-based quorum sensing (QS) in aerobic sludge granulation.Appl Microbiol Biotechnol. 2014 Sep;98(17):7623-32. doi: 10.1007/s00253-014-5815-3. Epub 2014 May 21. Appl Microbiol Biotechnol. 2014. PMID: 24846735
-
N-acyl homoserine lactone molecules assisted quorum sensing: effects consequences and monitoring of bacteria talking in real life.Arch Microbiol. 2021 Sep;203(7):3739-3749. doi: 10.1007/s00203-021-02381-9. Epub 2021 May 18. Arch Microbiol. 2021. PMID: 34002253 Review.
Cited by
-
Lactonase-mediated inhibition of quorum sensing largely alters phenotypes, proteome, and antimicrobial activities in Burkholderia thailandensis E264.Front Cell Infect Microbiol. 2023 Jun 2;13:1190859. doi: 10.3389/fcimb.2023.1190859. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37333853 Free PMC article.
-
Antimicrobial efficiency and cytocompatibility of resveratrol and naringin as chemical decontaminants on SLA surface.Microbiol Spectr. 2024 Oct 3;12(10):e0367923. doi: 10.1128/spectrum.03679-23. Epub 2024 Sep 6. Microbiol Spectr. 2024. PMID: 39240122 Free PMC article.
-
New Preventive Strategy against Oral Biofilm Formation in Caries-Active Children: An In Vitro Study.Antibiotics (Basel). 2023 Jul 31;12(8):1263. doi: 10.3390/antibiotics12081263. Antibiotics (Basel). 2023. PMID: 37627682 Free PMC article.
-
Silent signals: how N-acyl homoserine lactones drive oral microbial behaviour and health outcomes.Front Oral Health. 2024 Dec 5;5:1484005. doi: 10.3389/froh.2024.1484005. eCollection 2024. Front Oral Health. 2024. PMID: 39703871 Free PMC article. Review.
-
Quorum quenching by Est816: a novel approach to control Porphyromonas gingivalis pathogenicity.BMC Oral Health. 2025 Jul 16;25(1):1178. doi: 10.1186/s12903-025-06563-5. BMC Oral Health. 2025. PMID: 40671022 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

