Advanced characterization of surface-modified nanoparticles and nanofilled antibacterial dental adhesive resins
- PMID: 32555360
- PMCID: PMC7299952
- DOI: 10.1038/s41598-020-66819-8
Advanced characterization of surface-modified nanoparticles and nanofilled antibacterial dental adhesive resins
Abstract
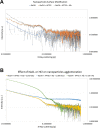
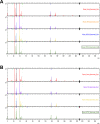
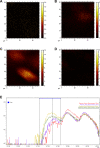
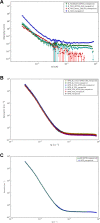
Nanotechnology can improve the performance of dental polymers. The objective of this study was to modify the surfaces of nanoparticles with silanes and proteins, characterize nanoparticles' agglomeration levels and interfaces between nanoparticles and the polymeric matrix. Undoped (n-TiO2), nitrogen-doped (N_TiO2) and nitrogen-fluorine co-doped titanium dioxide nanoparticles (NF_TiO2) were synthesized and subjected to surface modification procedures in preparation for Small-Angle X-Ray Scattering (SAXS) and Small-Angle Neutron Scattering (SANS) characterizations. Experimental adhesives were manually synthesized by incorporating 20% (v/v) of n-TiO2, N_TiO2 or NF_TiO2 (as-synthesized or surface-modified) into OptiBond Solo Plus (OPTB). Specimens (n = 15/group; d = 6.0 mm, t = 0.5 mm) of OPTB and experimental adhesives were characterized using Time-of-Flight Secondary Ion Mass Spectroscopy (ToF-SIMS), 2-D ToF-SIMS chemical imaging and SANS. SAXS results indicated that surface-modified nanoparticles displayed higher scattering intensities in a particle-size dependent manner. ToF-SIMS results demonstrated that nanoparticles' incorporation did not adversely impact the parental polymer. 2-D ToF-SIMS chemical imaging demonstrated the distribution of Ti+ and confirmed nitrogen-doping levels. SANS results confirmed nanoparticles' functionalization and revealed the interfaces between nanoparticles and the polymer matrix. Metaloxide nanoparticles were successfully fabricated, incorporated and covalently functionalized in a commercial dental adhesive resin, thereby supporting the utilization of nanotechnology in dentistry.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Shear Bond Strength and Color Stability of Novel Antibacterial Nanofilled Dental Adhesive Resins.Nanomaterials (Basel). 2022 Dec 20;13(1):1. doi: 10.3390/nano13010001. Nanomaterials (Basel). 2022. PMID: 36615911 Free PMC article.
-
Antibacterial dental adhesive resins containing nitrogen-doped titanium dioxide nanoparticles.Mater Sci Eng C Mater Biol Appl. 2018 Dec 1;93:931-943. doi: 10.1016/j.msec.2018.08.060. Epub 2018 Sep 1. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 30274130 Free PMC article.
-
Mechanical and antibacterial properties of an experimental flowable composite containing Nb2O5 and NF_TiO2 nanoparticles.J Mech Behav Biomed Mater. 2023 Jul;143:105919. doi: 10.1016/j.jmbbm.2023.105919. Epub 2023 May 25. J Mech Behav Biomed Mater. 2023. PMID: 37279637
-
Characterization of Experimental Nanoparticulated Dental Adhesive Resins with Long-Term Antibacterial Properties.Nanomaterials (Basel). 2022 Oct 24;12(21):3732. doi: 10.3390/nano12213732. Nanomaterials (Basel). 2022. PMID: 36364508 Free PMC article.
-
Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries.Nanomedicine (Lond). 2015 Mar;10(4):627-41. doi: 10.2217/nnm.14.191. Nanomedicine (Lond). 2015. PMID: 25723095 Free PMC article. Review.
Cited by
-
Shear Bond Strength and Color Stability of Novel Antibacterial Nanofilled Dental Adhesive Resins.Nanomaterials (Basel). 2022 Dec 20;13(1):1. doi: 10.3390/nano13010001. Nanomaterials (Basel). 2022. PMID: 36615911 Free PMC article.
-
Antibacterial Agents for Composite Resin Restorative Materials: Current Knowledge and Future Prospects.Cureus. 2024 Mar 29;16(3):e57212. doi: 10.7759/cureus.57212. eCollection 2024 Mar. Cureus. 2024. PMID: 38681374 Free PMC article. Review.
-
Aminosilane-Functionalized Zeolite Y in Pebax Mixed Matrix Hollow Fiber Membranes for CO2/CH4 Separation.Polymers (Basel). 2022 Dec 26;15(1):102. doi: 10.3390/polym15010102. Polymers (Basel). 2022. PMID: 36616452 Free PMC article.
-
Optimization of an ultra-bright real-time high-throughput renilla luciferase assay for antibacterial assessment of Streptococcus mutans biofilms.Dent Mater. 2024 Sep;40(9):1313-1321. doi: 10.1016/j.dental.2024.06.012. Epub 2024 Jun 13. Dent Mater. 2024. PMID: 38876827
-
Sorption, solubility and cytotoxicity of novel antibacterial nanofilled dental adhesive resins.Sci Rep. 2020 Aug 11;10(1):13503. doi: 10.1038/s41598-020-70487-z. Sci Rep. 2020. PMID: 32782299 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

