Simulating the human colorectal cancer microenvironment in 3D tumor-stroma co-cultures in vitro and in vivo
- PMID: 32555362
- PMCID: PMC7300090
- DOI: 10.1038/s41598-020-66785-1
Simulating the human colorectal cancer microenvironment in 3D tumor-stroma co-cultures in vitro and in vivo
Abstract
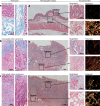
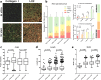
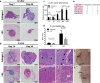
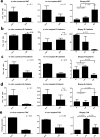
The tumor microenvironment (TME) plays a significant role in cancer progression and thus modeling it will advance our understanding of cancer growth dynamics and response to therapies. Most in vitro models are not exposed to intact body physiology, and at the same time, fail to recapitulate the extensive features of the tumor stroma. Conversely, animal models do not accurately capture the human tumor architecture. We address these deficiencies with biofabricated colorectal cancer (CRC) tissue equivalents, which are built to replicate architectural features of biopsied CRC tissue. Our data shows that tumor-stroma co-cultures consisting of aligned extracellular matrix (ECM) fibers and ordered micro-architecture induced an epithelial phenotype in CRC cells while disordered ECM drove a mesenchymal phenotype, similar to well and poorly differentiated tumors, respectively. Importantly, co-cultures studied in vitro, and upon implantation in mice, revealed similar tumor growth dynamics and retention of architectural features for 28 days. Altogether, these results are the first demonstration of replicating human tumor ECM architecture in ex vivo and in vivo cultures.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Khoo CY, Chai X, Quek R, Teo MCC, Goh BKP. Systematic review of current prognostication systems for primary gastrointestinal stromal tumors. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2018;44:388–394. doi: 10.1016/j.ejso.2017.12.006. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

