S100A6 is a critical regulator of hematopoietic stem cells
- PMID: 32555370
- PMCID: PMC7685984
- DOI: 10.1038/s41375-020-0901-2
S100A6 is a critical regulator of hematopoietic stem cells
Erratum in
-
Correction: S100A6 is a critical regulator of hematopoietic stem cells.Leukemia. 2020 Dec;34(12):3439. doi: 10.1038/s41375-020-0971-1. Leukemia. 2020. PMID: 32665696 Free PMC article.
Abstract
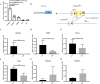
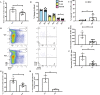
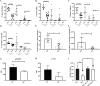
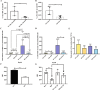
The fate options of hematopoietic stem cells (HSCs) include self-renewal, differentiation, migration, and apoptosis. HSCs self-renewal divisions in stem cells are required for rapid regeneration during tissue damage and stress, but how precisely intracellular calcium signals are regulated to maintain fate options in normal hematopoiesis is unclear. S100A6 knockout (KO) HSCs have reduced total cell numbers in the HSC compartment, decreased myeloid output, and increased apoptotic HSC numbers in steady state. S100A6KO HSCs had impaired self-renewal and regenerative capacity, not responding to 5-Fluorouracil. Our transcriptomic and proteomic profiling suggested that S100A6 is a critical HSC regulator. Intriguingly, S100A6KO HSCs showed decreased levels of phosphorylated Akt (p-Akt) and Hsp90, with an impairment of mitochondrial respiratory capacity and a reduction of mitochondrial calcium levels. We showed that S100A6 regulates intracellular and mitochondria calcium buffering of HSC upon cytokine stimulation and have demonstrated that Akt activator SC79 reverts the levels of intracellular and mitochondrial calcium in HSC. Hematopoietic colony-forming activity and the Hsp90 activity of S100A6KO are restored through activation of the Akt pathway. We show that p-Akt is the prime downstream mechanism of S100A6 in the regulation of HSC self-renewal by specifically governing mitochondrial metabolic function and Hsp90 protein quality.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

