Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine
- PMID: 32555376
- PMCID: PMC7880238
- DOI: 10.1038/s41573-020-0070-z
Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine
Abstract
The Hippo pathway is an evolutionarily conserved signalling pathway with key roles in organ development, epithelial homeostasis, tissue regeneration, wound healing and immune modulation. Many of these roles are mediated by the transcriptional effectors YAP and TAZ, which direct gene expression via control of the TEAD family of transcription factors. Dysregulated Hippo pathway and YAP/TAZ-TEAD activity is associated with various diseases, most notably cancer, making this pathway an attractive target for therapeutic intervention. This Review highlights the key findings from studies of Hippo pathway signalling across biological processes and diseases, and discusses new strategies and therapeutic implications of targeting this pathway.
Conflict of interest statement
Competing interests
K.-L.G. is a co-founder and has an equity interest in Vivace Therapeutics, Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies. A.D. is an employee of Genentech and shareholder at Roche.
Figures
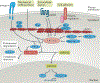



References
-
- Justice RW, Zilian O, Woods DF, Noll M & Bryant PJ The Drosophila tumor suppressor gene Warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534–546 (1995). - PubMed
-
- Kango-Singh M et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129, 5719–5730 (2002). - PubMed
-
- Pantalacci S, Tapon N & Leopold P The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5, 921–927 (2003). - PubMed
-
- Tapon N et al. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110, 467–478 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

