Molecular basis of β-arrestin coupling to formoterol-bound β1-adrenoceptor
- PMID: 32555462
- PMCID: PMC7115876
- DOI: 10.1038/s41586-020-2419-1
Molecular basis of β-arrestin coupling to formoterol-bound β1-adrenoceptor
Abstract
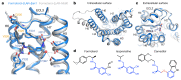
The β1-adrenoceptor (β1AR) is a G-protein-coupled receptor (GPCR) that couples1 to the heterotrimeric G protein Gs. G-protein-mediated signalling is terminated by phosphorylation of the C terminus of the receptor by GPCR kinases (GRKs) and by coupling of β-arrestin 1 (βarr1, also known as arrestin 2), which displaces Gs and induces signalling through the MAP kinase pathway2. The ability of synthetic agonists to induce signalling preferentially through either G proteins or arrestins-known as biased agonism3-is important in drug development, because the therapeutic effect may arise from only one signalling cascade, whereas the other pathway may mediate undesirable side effects4. To understand the molecular basis for arrestin coupling, here we determined the cryo-electron microscopy structure of the β1AR-βarr1 complex in lipid nanodiscs bound to the biased agonist formoterol5, and the crystal structure of formoterol-bound β1AR coupled to the G-protein-mimetic nanobody6 Nb80. βarr1 couples to β1AR in a manner distinct to that7 of Gs coupling to β2AR-the finger loop of βarr1 occupies a narrower cleft on the intracellular surface, and is closer to transmembrane helix H7 of the receptor when compared with the C-terminal α5 helix of Gs. The conformation of the finger loop in βarr1 is different from that adopted by the finger loop of visual arrestin when it couples to rhodopsin8. β1AR coupled to βarr1 shows considerable differences in structure compared with β1AR coupled to Nb80, including an inward movement of extracellular loop 3 and the cytoplasmic ends of H5 and H6. We observe weakened interactions between formoterol and two serine residues in H5 at the orthosteric binding site of β1AR, and find that formoterol has a lower affinity for the β1AR-βarr1 complex than for the β1AR-Gs complex. The structural differences between these complexes of β1AR provide a foundation for the design of small molecules that could bias signalling in the β-adrenoceptors.
Conflict of interest statement
Figures









Similar articles
-
Low affinity of beta1-adrenergic receptor for beta-arrestins explains the resistance to agonist-induced internalization.Life Sci. 2001 Apr 6;68(19-20):2251-7. doi: 10.1016/s0024-3205(01)01013-x. Life Sci. 2001. PMID: 11358334
-
Structure of the neurotensin receptor 1 in complex with β-arrestin 1.Nature. 2020 Mar;579(7798):303-308. doi: 10.1038/s41586-020-1953-1. Epub 2020 Jan 16. Nature. 2020. PMID: 31945771 Free PMC article.
-
Similarities and differences in the coupling of human beta1- and beta2-adrenoceptors to Gs(alpha) splice variants.Biochem Pharmacol. 2002 Jul 1;64(1):9-20. doi: 10.1016/s0006-2952(02)00924-3. Biochem Pharmacol. 2002. PMID: 12106601
-
Cryo-EM structures of GPCRs coupled to Gs, Gi and Go.Mol Cell Endocrinol. 2019 May 15;488:1-13. doi: 10.1016/j.mce.2019.02.006. Epub 2019 Mar 28. Mol Cell Endocrinol. 2019. PMID: 30930094 Review.
-
Structural features of β2 adrenergic receptor: crystal structures and beyond.Mol Cells. 2015;38(2):105-11. doi: 10.14348/molcells.2015.2301. Epub 2014 Dec 24. Mol Cells. 2015. PMID: 25537861 Free PMC article. Review.
Cited by
-
Discovery of Novel Trace Amine-Associated Receptor 5 (TAAR5) Antagonists Using a Deep Convolutional Neural Network.Int J Mol Sci. 2022 Mar 14;23(6):3127. doi: 10.3390/ijms23063127. Int J Mol Sci. 2022. PMID: 35328548 Free PMC article.
-
Heterotrimeric Gq proteins act as a switch for GRK5/6 selectivity underlying β-arrestin transducer bias.Nat Commun. 2022 Jan 25;13(1):487. doi: 10.1038/s41467-022-28056-7. Nat Commun. 2022. PMID: 35078997 Free PMC article.
-
Molecular insights into the biased signaling mechanism of the μ-opioid receptor.Mol Cell. 2021 Oct 21;81(20):4165-4175.e6. doi: 10.1016/j.molcel.2021.07.033. Epub 2021 Aug 24. Mol Cell. 2021. PMID: 34433090 Free PMC article.
-
Exploring GPCR conformational dynamics using single-molecule fluorescence.Methods. 2024 Jun;226:35-48. doi: 10.1016/j.ymeth.2024.03.011. Epub 2024 Apr 10. Methods. 2024. PMID: 38604413 Free PMC article. Review.
-
A small molecule enhances arrestin-3 binding to the β2-adrenergic receptor.Commun Chem. 2025 Jul 1;8(1):194. doi: 10.1038/s42004-025-01581-4. Commun Chem. 2025. PMID: 40593109 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

