Preparation of a phosphotyrosine-protein standard for use in semiquantitative western blotting with enhanced chemiluminescence
- PMID: 32555693
- PMCID: PMC7302481
- DOI: 10.1371/journal.pone.0234645
Preparation of a phosphotyrosine-protein standard for use in semiquantitative western blotting with enhanced chemiluminescence
Abstract
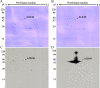
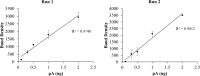
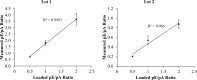
Protein tyrosine phosphorylation is key to activation of receptor tyrosine kinases (RTK) that drive development of some cancers. One challenge of RTK-targeted therapy is identification of those tumors that express non-mutated but activated RTKs. Phosphotyrosine (pTyr) RTK levels should be more predictive of the latter than expressed total protein. Western blotting (WB) with a pTyr antibody and enhanced chemiluminescence (ECL) detection is sufficiently sensitive to detect pTyr-RTKs in human tumor homogenates. Presentation of results by comparing WB images, however, is wanting. Here we describe the preparation of a new pTyr-protein standard, pTyr-ALK48-SB (pA), derived from a commercial anaplastic lymphoma kinase (ALK) recombinant fragment, and its use to quantify pTyr-epidermal growth factor receptor (pTyr-EGFR) in commercial A431 cell lysates. Linearity of one-dimensional (1D) WB plots of pA band density versus load as well as its lower level of detection (0.1 ng, 2 fmole) were determined for standardized conditions. Adding pA to two lots of A431 cell lysates with high and low pTyr-EGFR allowed normalization and quantification of the latter by expressing results as density ratios for both 1D and 2D WB. This approach is semi-quantitative because unknown RTKs may be outside the linear range of detection. Semiquantitative ratios are an improvement over comparisons of images without a reference standard and facilitate comparisons between samples.
Conflict of interest statement
The authors have read the journal's policy and the authors of this manuscript have the following competing interests: NK, JJ, GP, MH, AD, and SC are paid employees of Kendrick Labs, Inc. A US Patent has been applied for by Kendrick Labs, Inc. entitled “Protein Standard Compositions and Methods of Making and Using The Same” [U.S. Patent Application No. is: 15/808,006]. However, this does not alter our adherence to all PLOS ONE policies on sharing data and materials.
Figures





References
-
- Weinberg RA. The biology of cancer. Second edition ed. New York: Garland Science, Taylor & Francis Group; 2014. xx, 876, A 6, G 30, I 28 pages p.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

