Gut dysbiosis modulates the immune response to factor VIII in murine hemophilia A
- PMID: 32556285
- PMCID: PMC7322952
- DOI: 10.1182/bloodadvances.2019001144
Gut dysbiosis modulates the immune response to factor VIII in murine hemophilia A
Abstract
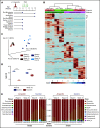
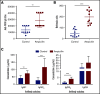
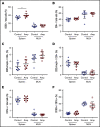
The development of neutralizing FVIII antibodies is the most serious complication of hemophilia A treatment. The currently known patient- and treatment-related risk factors for inhibitor development do not accurately predict this adverse event in all patients. The composition of the gut microbiota has been shown to influence immune-mediated diseases at distant anatomical sites (eg, lungs, brain, and joints). We demonstrate that a disrupted gut microbiota can be created in a mouse model of hemophilia A using a broad-spectrum antibiotic. Under controlled conditions, this sustained dysbiosis was associated with an increase in splenic B cells and the development of higher titer, FVIII-specific immunoglobulin G antibodies after FVIII challenge. Splenic and mesenteric lymph node cytokines, T cells, and dendritic cells were unaffected before administration of FVIII. However, the immune transcriptome of both aforementioned secondary lymphoid organs was significantly modified. Short-chain fatty acids (SCFAs), which are immunomodulatory microbial metabolites, were depleted in cecal contents of the dysbiotic mice. Furthermore, supplementation of the drinking water with butyrate, the most immunologically active SCFA, successfully achieved attenuation of the FVIII immune response. Collectively, data from this exploratory study suggest that the composition of the gut microbiota alters the FVIII immune response via the action of specific microbial metabolites on the immune cell transcriptome and that oral supplementation with butyrate effectively reduces the FVIII immune response.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: D.L. received research support from Bayer, Biogen, Biomarin, CSL-Behring, and Octapharma. The remaining authors declare no competing financial interests.
Figures


Similar articles
-
Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations.J Physiol. 2018 Oct;596(20):4923-4944. doi: 10.1113/JP276431. Epub 2018 Aug 28. J Physiol. 2018. PMID: 30066368 Free PMC article.
-
Blockade of CD40/CD40 ligand interactions prevents induction of factor VIII inhibitors in hemophilic mice but does not induce lasting immune tolerance.Thromb Haemost. 2001 Dec;86(6):1345-52. Thromb Haemost. 2001. PMID: 11776297
-
Role of CD154 in the secondary immune response: the reduction of pre-existing splenic germinal centers and anti-factor VIII inhibitor titer.Eur J Immunol. 2000 Sep;30(9):2548-54. doi: 10.1002/1521-4141(200009)30:9<2548::AID-IMMU2548>3.0.CO;2-H. Eur J Immunol. 2000. PMID: 11009088
-
Tolerating Factor VIII: Recent Progress.Front Immunol. 2020 Jan 10;10:2991. doi: 10.3389/fimmu.2019.02991. eCollection 2019. Front Immunol. 2020. PMID: 31998296 Free PMC article. Review.
-
Review of immune tolerance induction in hemophilia A.Blood Rev. 2018 Jul;32(4):326-338. doi: 10.1016/j.blre.2018.02.003. Epub 2018 Feb 15. Blood Rev. 2018. PMID: 29482894 Review.
Cited by
-
Mice possess a more limited natural antihuman factor VIII antibody repertoire than humans that is produced disproportionately by marginal zone B cells.J Thromb Haemost. 2024 Jan;22(1):76-89. doi: 10.1016/j.jtha.2023.08.033. Epub 2023 Sep 6. J Thromb Haemost. 2024. PMID: 37678547 Free PMC article.
References
-
- Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361(9371):1801-1809. - PubMed
-
- Soucie JM, Evatt B, Jackson D; The Hemophilia Surveillance System Project Investigators . Occurrence of hemophilia in the United States. Am J Hematol. 1998;59(4):288-294. - PubMed
-
- Key NS, Negrier C. Coagulation factor concentrates: past, present, and future. Lancet. 2007;370(9585):439-448. - PubMed
-
- Hay CRM. The epidemiology of factor VIII inhibitors. Haemophilia. 2006;12(suppl 6):23-28, discussion 28-29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

