T cell immunity rather than antibody mediates cross-protection against Zika virus infection conferred by a live attenuated Japanese encephalitis SA14-14-2 vaccine
- PMID: 32556415
- PMCID: PMC7347694
- DOI: 10.1007/s00253-020-10710-z
T cell immunity rather than antibody mediates cross-protection against Zika virus infection conferred by a live attenuated Japanese encephalitis SA14-14-2 vaccine
Abstract
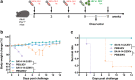
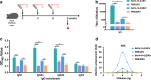
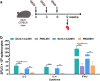
Zika virus (ZIKV) and Japanese encephalitis virus (JEV) are closely related to mosquito-borne flaviviruses. Japanese encephalitis (JE) vaccine SA14-14-2 has been in the Chinese national Expanded Program on Immunization since 2007. The recent recognition of severe disease syndromes associated with ZIKV, and the identification of ZIKV from mosquitoes in China, prompts an urgent need to investigate the potential interaction between the two. In this study, we showed that SA14-14-2 is protective against ZIKV infection in mice. JE vaccine SA14-14-2 triggered both Th1 and Th2 cross-reactive immune responses to ZIKV; however, it was cellular immunity that predominantly mediated cross-protection against ZIKV infection. Passive transfer of immune sera did not result in significant cross-protection but did mediate antibody-dependent enhancement in vitro, though this did not have an adverse impact on survival. This study suggests that the SA14-14-2 vaccine can protect against ZIKV through a cross-reactive T cell response. This is vital information in terms of ZIKV prevention or precaution in those ZIKV-affected regions where JEV circulates or SA14-14-2 is in widespread use, and opens a promising avenue to develop a novel bivalent vaccine against both ZIKV and JEV. KEY POINTS: • JEV SA14-14-2 vaccine conferred cross-protection against ZIKV challenge in mice. • T cell immunity rather than antibody mediated the cross-protection. • It provides important information in terms of ZIKV prevention or precaution.
Keywords: Cross-protection; Cross-reactivity; Japanese encephalitis virus; SA14-14-2 vaccine; T cell; Zika virus.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Partial protective efficacy of the current licensed Japanese encephalitis live vaccine against the emerging genotype I Japanese encephalitis virus isolated from sheep.Front Immunol. 2025 Feb 13;16:1513261. doi: 10.3389/fimmu.2025.1513261. eCollection 2025. Front Immunol. 2025. PMID: 40018033 Free PMC article.
-
Cellular Immune Responses to Live Attenuated Japanese Encephalitis (JE) Vaccine SA14-14-2 in Adults in a JE/Dengue Co-Endemic Area.PLoS Negl Trop Dis. 2017 Jan 30;11(1):e0005263. doi: 10.1371/journal.pntd.0005263. eCollection 2017 Jan. PLoS Negl Trop Dis. 2017. PMID: 28135273 Free PMC article. Clinical Trial.
-
Japanese encephalitis virus E protein domain III immunization mediates cross-protection against Zika virus in mice via antibodies and CD8+T cells.Virus Res. 2024 Jul;345:199376. doi: 10.1016/j.virusres.2024.199376. Epub 2024 Apr 20. Virus Res. 2024. PMID: 38643856 Free PMC article.
-
Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities.Vaccine. 2010 May 7;28(21):3635-41. doi: 10.1016/j.vaccine.2010.02.105. Epub 2010 Mar 11. Vaccine. 2010. PMID: 20226891 Review.
-
Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease.Hum Vaccin Immunother. 2017 Jun 3;13(6):1-18. doi: 10.1080/21645515.2017.1285472. Epub 2017 Feb 22. Hum Vaccin Immunother. 2017. PMID: 28301270 Free PMC article. Review.
Cited by
-
Japanese Encephalitis Vaccine Generates Cross-Reactive Memory T Cell Responses to Zika Virus in Humans.J Trop Med. 2022 Nov 19;2022:8379286. doi: 10.1155/2022/8379286. eCollection 2022. J Trop Med. 2022. PMID: 36444358 Free PMC article.
-
Widespread interspecific phylogenetic tree incongruence between mosquito-borne and insect-specific flaviviruses at hotspots originally identified in Zika virus.Virus Evol. 2022 Apr 18;8(1):veac027. doi: 10.1093/ve/veac027. eCollection 2022. Virus Evol. 2022. PMID: 35591877 Free PMC article.
-
Zika virus-like particle vaccine fusion loop mutation increases production yield but fails to protect AG129 mice against Zika virus challenge.PLoS Negl Trop Dis. 2022 Jul 6;16(7):e0010588. doi: 10.1371/journal.pntd.0010588. eCollection 2022 Jul. PLoS Negl Trop Dis. 2022. PMID: 35793354 Free PMC article.
-
Cross-Reactive Immunity among Five Medically Important Mosquito-Borne Flaviviruses Related to Human Diseases.Viruses. 2022 Jun 2;14(6):1213. doi: 10.3390/v14061213. Viruses. 2022. PMID: 35746683 Free PMC article. Review.
-
The Japanese Encephalitis Antigenic Complex Viruses: From Structure to Immunity.Viruses. 2022 Oct 8;14(10):2213. doi: 10.3390/v14102213. Viruses. 2022. PMID: 36298768 Free PMC article. Review.
References
-
- Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Loriere E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, England P, Stiasny K, Mongkolsapaya J, Heinz FX, Screaton GR, Rey FA. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature. 2016;536(7614):48–53. doi: 10.1038/nature18938. - DOI - PubMed
-
- Breitbach ME, Newman CM, Dudley DM, Stewart LM, Aliota MT, Koenig MR, Shepherd PM, Yamamoto K, Crooks CM, Young G, Semler MR, Weiler AM, Barry GL, Heimsath H, Mohr EL, Eichkoff J, Newton W, Peterson E, Schultz-Darken N, Permar SR, Dean H, Capuano S, 3rd, Osorio JE, Friedrich TC, O'Connor DH. Primary infection with dengue or Zika virus does not affect the severity of heterologous secondary infection in macaques. PLoS Pathog. 2019;15(8):e1007766. doi: 10.1371/journal.ppat.1007766. - DOI - PMC - PubMed
-
- Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, Mongkolsapaya J, Screaton GR. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016;17(9):1102–1108. doi: 10.1038/ni.3515. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

