Placental Production of Eicosanoids and Sphingolipids in Women Who Developed Preeclampsia on Low-Dose Aspirin
- PMID: 32557282
- PMCID: PMC7606383
- DOI: 10.1007/s43032-020-00234-2
Placental Production of Eicosanoids and Sphingolipids in Women Who Developed Preeclampsia on Low-Dose Aspirin
Abstract
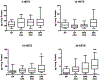
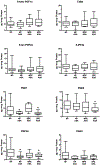
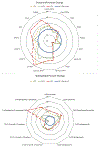
Low-dose aspirin, which selectively inhibits thromboxane synthesis, is now standard of care for the prevention of preeclampsia in at risk women, but some women still develop preeclampsia despite an aspirin regimen. To explore the "aspirin failures," we undertook a comprehensive evaluation of placental lipids to determine if abnormalities in non-aspirin sensitive lipids might help explain why some women on low-dose aspirin develop preeclampsia. We studied placentas from women with normal pregnancies and women with preeclampsia. Placental villous explants were cultured and media analyzed by mass spectrometry for aspirin-sensitive and non-aspirin-sensitive lipids. In women who developed severe preeclampsia and delivered preterm, there were significant elevations in non-aspirin-sensitive lipids with biologic actions that could cause preeclampsia. There were significant increases in 15- and 20-hydroxyeicosatetraenoic acids and sphingolipids: D-e-C18:0 ceramide, D-e-C18:0 sphingomyelin, D-e-sphingosine-1-phosphate, and D-e-sphinganine-1-phosphate. With regard to lipids sensitive to aspirin, there was no difference in placental production of thromboxane or prostacyclin, but prostaglandins were lower. There was no difference for isoprostanes, but surprisingly, anti-inflammatory omega 3 and 6 PUFAs were increased. In total, 10 of 30 eicosanoids and 5 of 42 sphingolipids were abnormal in women with severe early onset preeclampsia. Lipid changes in women with mild preeclampsia who delivered at term were of lesser magnitude with few significant differences. The placenta produces many aspirin-sensitive and non-aspirin-sensitive lipids. Abnormalities in eicosanoids and sphingolipids not sensitive to aspirin might explain why some aspirin-treated women develop preeclampsia.
Keywords: Eicosanoids; Low-dose aspirin; Placenta; Preeclampsia; Sphingolipids.
Conflict of interest statement
Figures

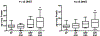

References
-
- Wallenburg HCS, Makovitz JW, Dekker GA, Rotmans P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensinsensitive primigravidae. Lancet. 1986;1(8471):1–3. - PubMed
-
- Walsh SW. Preeclampsia: An imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152(3):335–40. - PubMed
-
- Bunting S, Moncada S, Vane JR. The prostacyclin--thromboxane A2 balance: pathophysiological and therapeutic implications. Br Med Bull. 1983;39(3):271–6. - PubMed
-
- Lewis HD Jr., Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE 3rd et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1983;309(7):396–403. doi:10.1056/nejm198308183090703. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

