Pitavastatin for lowering lipids
- PMID: 32557581
- PMCID: PMC7387421
- DOI: 10.1002/14651858.CD012735.pub2
Pitavastatin for lowering lipids
Abstract
Background: Pitavastatin is the newest statin on the market, and the dose-related magnitude of effect of pitavastatin on blood lipids is not known.
Objectives: Primary objective To quantify the effects of various doses of pitavastatin on the surrogate markers: LDL cholesterol, total cholesterol, HDL cholesterol and triglycerides in participants with and without cardiovascular disease. To compare the effect of pitavastatin on surrogate markers with other statins. Secondary objectives To quantify the effect of various doses of pitavastatin on withdrawals due to adverse effects. SEARCH METHODS: The Cochrane Hypertension Information Specialist searched the following databases for trials up to March 2019: the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 2, 2019), MEDLINE (from 1946), Embase (from 1974), the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. We also contacted authors of relevant papers regarding further published and unpublished work. The searches had no language restrictions.
Selection criteria: RCT and controlled before-and-after studies evaluating the dose response of different fixed doses of pitavastatin on blood lipids over a duration of three to 12 weeks in participants of any age with and without cardiovascular disease.
Data collection and analysis: Two review authors independently assessed eligibility criteria for studies to be included, and extracted data. We entered data from RCT and controlled before-and-after studies into Review Manager 5 as continuous and generic inverse variance data, respectively. Withdrawals due to adverse effects (WDAE) information was collected from the RCTs. We assessed all included trials using the Cochrane 'Risk of bias' tool under the categories of allocation (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential sources of bias.
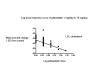
Main results: Forty-seven studies (five RCTs and 42 before-and-after studies) evaluated the dose-related efficacy of pitavastatin in 5436 participants. The participants were of any age with and without cardiovascular disease, and pitavastatin effects were studied within a treatment period of three to 12 weeks. Log dose-response data over doses of 1 mg to 16 mg revealed strong linear dose-related effects on blood total cholesterol and LDL cholesterol and triglycerides. There was no dose-related effect of pitavastatin on blood HDL cholesterol, which was increased by 4% on average by pitavastatin. Pitavastatin 1 mg/day to 16 mg/day reduced LDL cholesterol by 33.3% to 54.7%, total cholesterol by 23.3% to 39.0% and triglycerides by 13.0% to 28.1%. For every two-fold dose increase, there was a 5.35% (95% CI 3.32 to 7.38) decrease in blood LDL cholesterol, a 3.93% (95% CI 2.35 to 5.50) decrease in blood total cholesterol and a 3.76% (95% CI 1.03 to 6.48) decrease in blood triglycerides. The certainty of evidence for these effects was judged to be high. When compared to other statins for its effect to reduce LDL cholesterol, pitavastatin is about 6-fold more potent than atorvastatin, 1.7-fold more potent than rosuvastatin, 77-fold more potent than fluvastatin and 3.3-fold less potent than cerivastatin. For the placebo group, there were no participants who withdrew due to an adverse effect per 109 subjects and for all doses of pitavastatin, there were three participants who withdrew due to an adverse effect per 262 subjects.
Authors' conclusions: Pitavastatin lowers blood total cholesterol, LDL cholesterol and triglyceride in a dose-dependent linear fashion. Based on the effect on LDL cholesterol, pitavastatin is about 6-fold more potent than atorvastatin, 1.7-fold more potent than rosuvastatin, 77-fold more potent than fluvastatin and 3.3-fold less potent than cerivastatin. There were not enough data to determine risk of withdrawal due to adverse effects due to pitavastatin.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Stephen P Adams: None known.
Nima Alaei: None known.
James M Wright: None known.
Figures




































Update of
References
References to studies included in this review
Braamskamp 2015 {published data only}
-
- Daniels SR. Pitavastatin in children. Journal of Pediatrics 2015;167(2):219-21. [DOI: ]
-
- World Health Organization. Investigating pitavastatin for high cholesterol in children [A double-blind, randomised, placebo-controlled, parallel-group, 12-week study of pitavastatin in high-risk hyperlipidaemia in childhood P/266/2011, P267/2011, P268/2011 - PASCAL 401]. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011-004964-32-NL first received 9 February 2012. [WHO 2020]
Budinski 2009 {published data only}
-
- Budinski D, Arneson V, Hounslow N, Gratsiansky N. Pitavastatin compared with atorvastatin in primary hypercholesterolemia or combined dyslipidemia. Clinical Lipidology 2009;4(3):291-302. [EMBASE: 354734823]
-
- Budinski D. Study comparing pitavastatin and atorvastatin in patients with type II diabetes mellitus and combined dyslipidemia. ClinicalTrials.gov . [CLINICALTRIALS.GOV ID: NCT00309751]
-
- Study to compare the efficacy of pitavastatin with that of atorvastatin in lowering cholesterol levels. ClinicalTrials.gov . [CLINICALTRIALS.GOV ID: NCT00249249]
-
- Study to compare the efficacy of pitavastatin with that of atorvastatin in lowering cholesterol levels. ICH Good Clinical Practice Clinical Trials Registry . [ICH CTR ID: NCT00249249]
Chen 2012 {published data only}
-
- Chen L, Qu P, Lou D, Liu Q, Wang J, Zhang C. Effect of pitavastatin on high-sensitive c-reactive protein levels in patients with hypertension [Pǐ fá tātīng duì gāo xiěyā huànzhě gāomǐn c fǎnyìng dànbái shuǐpíng de yǐngxiǎng]. Zhongguo xin yao yu lin chuang za zhi [Chinese Journal of New Drugs and Clinical Remedies] 2012;31(6):328-31.
Chen 2015 {published data only}
-
- Chen H, Zhang Y, Wei X, Han Z, Liu Z, Liu S. Clinical observation of pitavastatin in the treatment of hyperlipidemia [pi fa ta ting zhi liao gao zhi xue zheng lin chuang liao xiao guan cha]. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease 2015;13(06):822-4. [DOI: 10.3969/j.issn.1672-1349.2015.06.044] - DOI
Eriksson 2011 {published data only}
-
- EUCTR2005 001037 15. Study of pitavastatin 4 mg vs simvastatin 40 mg (following up-titration) in patients with primary hypercholesterolema or combined dyslipidemia and 2 or more risk factors for coronary heart disease. EU Clinical Trials Register/show/EUCTR2005 001037 15 (first received 29 December 2005). [EUCTR2005 001037 15]
-
- Good Clinical Practice Network. Study to compare the efficacy and safety of pitavastatin and simvastatin [Study of pitavastatin 4 mg vs simvastatin 40 mg (following up-titration) in patients with primary hypercholesterolema or combined dyslipidemia and 2 or more risk factors for coronary heart disease]. Good clinical practice network/show/NCT00309738 (first received September 2005). [URL: ichgcp.net/clinical-trials-registry/NCT00309738]
-
- NCT00309738. Study to compare the efficacy and safety of pitavastatin and simvastatin [Study of pitavastatin vs. simvastatin (following up-titration) in patients with primary hypercholesterolemia or combined dyslipidemia and 2 or more risk factors for coronary heart disease]. clinicaltrials.gov/show/NCT00309738 (first received September 2005). [NCT00309738]
Gumprecht 2011 {published data only}
-
- Center for Drug Evaluation and Research. Active-controlled study with atorvastatin in patients with type II diabetes mellitus (NK-104-305) [Study of pitavastatin 4 mg vs atorvastatin 20 mg (following up-titration) in subjects with type II diabetes mellitus and combined dyslipidemia [NK-104-305]]. FDA Open Trials/show/NK-104-305 first received 28 February 2012:21-2. [www.documentcloud.org/documents/3199241.html]
-
- Gumprecht J, Gosho M, Budinski D, Hounslow N. Comparative long-term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20-40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes, Obesity & Metabolism 2011;13(11):1047-55. [DOI: 10.1111/j.1463-1326.2011.01477.x] - DOI - PubMed
HaeKim 2018 {published data only}
Han 2012 {published data only}
-
- Han KH, Rha SW, Kang HJ, Bae JW, Choi BJ, Choi SY, et al. Evaluation of short-term safety and efficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: pitch study (pitavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). Journal of Clinical Lipidology 2012;6(4):340-51. [DOI: 10.1016/j.jacl.2012.01.009] - DOI - PubMed
-
- NCT01166633. Efficacy and safety study of pitavastatin versus atorvastatin to treat hypercholesterolemia (pitch) [A randomized, open label, dose titration study to evaluate the effect of pitavastatin versus atorvastatin in patients with hypercholesterolemia and mild to moderate hepatic damage]. clinicaltrials.gov/show/NCT01166633 (first received June 2009). [NCT01166633]
Harada Shiba 2016 {published data only}
Huang 2012 {published data only}
-
- Huang ZQ, Wu YT, Wang R, Huang W, Chen L. Effects of pitavastatin and atorvastatin on blood lipids and blood glucose in elderly type 2 diabetic patients [Pǐ fá tātīng jí ā tuō fá tātīng duì lǎonián 2 xíng tángniàobìng huànzhě xuèzhī, xiětáng de yǐngxiǎng]. Zhongguo xin yao yu lin chuang za zhi [Chinese Journal of New Drugs and Clinical Remedies] 2012;31(10):614-7. [CENTRAL: CN-00972987]
Ikegami 2012 {published data only}
Kajinami 2000 {published data only}
-
- Kajinami K, Koizumi J, Ueda K, Miyamoto S, Takegoshi T, Mabuchi H, Hokuriku nk-104 study Group. Effects of nk-104, a new hydroxymethylglutaryl-coenzyme reductase inhibitor, on low-density lipoprotein cholesterol in heterozygous familial hypercholesterolemia. American Journal of Cardiology 2000;85(2):178-83. [MEDLINE: ] - PubMed
Kakuda 2013 {published data only}
-
- Kakuda H, Kobayashi J, Nakato M, Takekoshi N. Short-term effect of pitavastatin treatment on glucose and lipid metabolism and oxidative stress in fasting and postprandial state using a test meal in Japanese men. Cholesterol 2013;2013(Article ID 314170):1-7. [DOI: 10.1155/2013/314170] - DOI - PMC - PubMed
Lee 2007 {published data only}
-
- Lee SH, Chung N, Kwan J, Kim DI, Kim WH, Kim CJ, et al. Comparison of the efficacy and tolerability of pitavastatin and atorvastatin: an 8-week, multicenter, randomized, open-label, dose-titration study in Korean patients with hypercholesterolemia. Clinical Therapeutics 2007;29(11):2365-73. [MEDLINE: ] - PubMed
Liu 2013 {published data only}
-
- Lin LY, Huang CC, Chen JS, Wu TC, Leu HB, Huang PH, et al. Effects of pitavastatin versus atorvastatin on the peripheral endothelial progenitor cells and vascular endothelial growth factor in high-risk patients: a pilot prospective, double-blind, randomized study. Cardiovascular Diabetology 2014;13:111. [DOI: 10.1186/s12933-014-0111-1] - DOI - PMC - PubMed
-
- Liu PY, Lin LY, Lin HJ, Hsia CH, Hung YR, Yeh HI, et al. Pitavastatin and atorvastatin double-blind randomized comparative study among high-risk patients, including those with type 2 diabetes mellitus, in Taiwan (papago-t study). PLOS One 2013;8(10):e76298 [erratum appears in PLOS One. 2014;9(11):e114175]. [DOI: 10.1371/journal.pone.0076298] - DOI - PMC - PubMed
-
- NCT01386853. Efficacy and safety of pitavastatin and atorvastatin in high risk hypercholesterolemic patients [A 12-week, randomized, multicenter, double-blind, active-controlled, non-inferiority study to compare the efficacy and safety of pitavastatin and atorvastatin in high risk hypercholesterolemic patients]. clinicaltrials.gov/show/NCT01386853 (first received July 2011). [NCT01386853]
Majima 2007 {published data only}
-
- Majima T, Shimatsu A, Komatsu Y, Satoh N, Fukao A, Ninomiya K. Short-term effects of pitavastatin on biochemical markers of bone turnover in patients with hypercholesterolemia. Internal Medicine 2007;46(24):1967-73. [MEDLINE: ] - PubMed
Mao 2012 {published data only}
-
- Mao Y, Yu JM, Zhan YQ, Hu DY, Ding RJ, Zhang F, et al. Safety and efficacy of pitavastatin in patients with hypercholesterolemia: a multicenter study [Pǐ fá tātīng zhìliáo gāo dǎngùchún xiě zhèng ānquán xìng hé yǒuxiào xìng de duō zhōngxīn guānchá]. Chung Hua I Hsueh Tsa Chih [Chinese Medical Journal (Taipei)] 2012;92(14):968-73. [MEDLINE: ] - PubMed
-
- Mao Y, Yu JM, Zhang F, Hu DY, Ding RJ, Zhan YQ, et al. The effect of pitavastatin on blood glucose and its efficacy in diabetic patients with hypercholesterolemia [Pǐ fá tātīng duì tángniàobìng gāo dǎngùchún xiě zhèng huànzhě xiětáng de yǐngxiǎng jí qí liáoxiào]. Chung Hua Nei Ko Tsa Chih [Chinese Journal of Internal Medicine] 2012;51(7):508-12. [MEDLINE: ] - PubMed
-
- Wang C. Clinical study on coronary heart disease. Chinese Medical Journal 2014;127(Suppl 2):49-54. [EMBASE: 2015362190]
Motomura 2009 {published data only}
-
- Motomura T, Okamoto M, Kitamura T, Yamamoto H, Otsuki M, Asanuma N, et al. Effects of pitavastatin on serum lipids and high sensitivity c-reactive protein in type 2 diabetic patients. Journal of Atherosclerosis & Thrombosis 2009;16(5):546-52. [MEDLINE: ] - PubMed
Nakamura 2008 {published data only}
-
- Nakamura T, Obata JE, Kitta Y, Takano H, Kobayashi T, Fujioka D, et al. Rapid stabilization of vulnerable carotid plaque within 1 month of pitavastatin treatment in patients with acute coronary syndrome. Journal of Cardiovascular Pharmacology 2008;51(4):365-71. [MEDLINE: ] - PubMed
NK‐104.202 2013 {published data only}
-
- Australian Government. Australian public assessment report for pitavastatin. Australian Government Department of Health Therapeutics Goods Administration 2013:49-52. [URL: www.tga.gov.au/sites/default/files/auspar-pitavastatin-130902.pdf]
-
- Chowdhury IN, Gortler D. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4 mg, and 8 mg compared to placebo in patients with primary hypercholesterolaema [HEC/NK98402N/NK-104.202]. Center for Drug Evaluation and Research Application Number 22-363 Medical Reviews 2009:39-45. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_MedR_P1.pdf]
-
- Mahayni H, Marroum P. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4 mg, and 8 mg compared to placebo in patients with primary hypercholesterolaema [HEC/NK98402N/NK-104.202]. Center for Drug Evaluation and Research Application Number 22-363 Clinical Pharmacology and Biopharmaceutics Review(s) 2009;Part 2:102. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_ClinPharmR_P2...]
-
- Min M, Lin K, Elmore C, Johnson K. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4 mg, and 8 mg compared to placebo in patients with primary hypercholesterolaema;[HEC/NK98402N/NK-104.202]. Center for Drug Evaluation and Research Application Number 22-363 Statistical Review(s) 2009:28. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_StatR.pdf]
NK‐104.203 2013 {published data only}
-
- Australian Government. Australian public assessment report for pitavastatin. Australian Government Department of Health Therapeutics Goods Administration 2013:49-52. [URL: www.tga.gov.au/sites/default/files/auspar-pitavastatin-130902.pdf]
-
- Chowdhury IN, Gortler D. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4 mg, and 8 mg compared to placebo in patients with primary mixed or combined hyperlipidaemia [HEC/NK98402N/NK-104.203]. Center for Drug Evaluation and Research Application Number: 22-363 Medical Review(s) 2009:39-45. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_MedR_P1.pdf]
-
- Mahayni H, Marroum P. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4mg, and 8 mg compared to placebo in patients with primary mixed or combined hyperlipidaemia; [HEC/NK98402N/NK-104.203]. Center for Drug Evaluation and Research Application Number: 22-363 Clinical Pharmacology and Biopharmaceutics Review(s) 2009;Part 2:102. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_ClinPharmR_P2...]
-
- Min M, Lin K, Elmore C, Johnson K. A multinational, multicenter randomised, double-blind, parallel-group, dose ranging study to evaluate the efficacy and safety of NK-104 1 mg, 2 mg, 4mg, and 8 mg compared to placebo in patients with primary mixed or combined hyperlipidaemia;[HEC/NK98402N/NK-104.203]. Center for Drug Evaluation and Research Application Number: 22-363 Statistical Review(s) 2009:28. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_StatR.pdf]
NK‐104.209 2013 {published data only}
-
- Australian Government. Australian public assessment report for pitavastatin. Australian Government Department of Health Therapeutics Goods Administration 2013:52-53. [URL: www.tga.gov.au/sites/default/files/auspar-pitavastatin-130902.pdf]
-
- Mahayni H, Marroum P. A dose ranging study of NK-104 in patients with primary hypercholesterolema; [NK-104-209]. Center for Drug Evaluation and Research Application Number: 22-363 Clinical Pharmacology and Biopharmaceutics Review(s) 2009;Part 2:102. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_ClinPharmR_P2...]
-
- Min M, Lin K, Elmore C, Johnson K. A dose ranging study of NK-104 in patients with primary hypercholesterolema; [NK-104-209]. Center for Drug Evaluation and Research Application Number: 22-363 Statistical Review(s) 2009:28. [URL: www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_StatR.pdf]
Noji 2002 {published data only}
-
- Mabuchi H, Koizumi J, Kajinami K, Miyamoto S, Takegoshi T. Long-term effects of NK-104, a new HMG-CoA reductase inhibitor, in patients with heterozygous familial hypercholesterolemia. Rinsho Iyaku 2001;17(6):915-43.
-
- Noji Y, Higashikata T, Inazu A, Nohara A, Ueda K, Miyamoto S, et al. Long-term treatment with pitavastatin (nk-104), a new HMG-CoA reductase inhibitor, of patients with heterozygous familial hypercholesterolemia. Atherosclerosis 2002;163(1):157-64. [MEDLINE: ] - PubMed
Nozue 2008 {published data only}
-
- Nozue T, Michishita I, Ito Y, Hirano T. Effects of statin on small dense low-density lipoprotein cholesterol and remnant-like particle cholesterol in heterozygous familial hypercholesterolemia. Journal of Atherosclerosis & Thrombosis 2008;15(3):146-53. [MEDLINE: ] - PubMed
Ohbayashi 2009 {published data only}
-
- Ohbayashi H, Miyazawa C, Miyamoto K, Sagara M, Yamashita T, Onda R. Pitavastatin improves plasma pentraxin 3 and arterial stiffness in atherosclerotic patients with hypercholesterolemia. Journal of Atherosclerosis & Thrombosis 2009;16(4):490-500. [MEDLINE: ] - PubMed
Ose 2009 {published data only}
-
- Center for Drug Evaluation and Research. Study to compare the efficacy and safety of pitavastatin and simvastatin [Study of pitavastatin 2 mg vs. simvastatin 20 mg and pitavastatin 4 mg (following up-titration) in subjects with primary hypercholesterolemia or combined dyslipidemia [NK-104-302]]. FDA Open Trials/show/NK-104-302 (first received 1 October 2008). [www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_MedR_P1.pdf]
-
- EUCTR2005-001033-15. Study of pitavastatin 2 mg vs. simvastatin 20 mg and pitavastatin 4 mg vs. simvastatin 40 mg (following up-titration) in patients with primary hypercholesterolemia or combined dyslipidemia. EU Clinical Trials Register/show/EUCTR2005-001033-15 (first received 1 August 2005). [EUCTR2005-001033-15]
-
- NCT00309777. Study to compare the efficacy and safety of pitavastatin and simvastatin [Study of pitavastatin vs. simvastatin (following up-titration) in patients with primary hypercholesterolemia or combined dyslipidemia]. clinicaltrials.gov/show/NCT00309777 (first received 1 July 2011). [NCT00309777]
-
- Ose L, Budinski D, Hounslow N, Arneson V. Comparison of pitavastatin with simvastatin in primary hypercholesterolaemia or combined dyslipidaemia. Current Medical Research and Opinion 2009;25(11):2755-64 [erratum appears in Current Medical Research and Opinion. 2010;26(5):1046 Note: dosage error in article text]. [DOI: 10.1185/03007990903290886] - DOI - PubMed
Park 2005 {published data only}
-
- Park S, Kang HJ, Rim SJ, Ha JW, Oh BH, Chung N, et al. A randomized, open-label study to evaluate the efficacy and safety of pitavastatin compared with simvastatin in Korean patients with hypercholesterolemia. Clinical Therapeutics 2005;27(7):1074-82. [MEDLINE: ] - PubMed
PREVAIL‐US 2016 {published data only}
-
- Kryzhanovski V, Morgan R, Sponseller C, Davidson M. Pitavastatin 4 mg provides significantly greater reduction in LDL-C compared to pravastatin 40 mg with neutral effects on glucose metabolism: prespecified safety analysis from the short-term phase 4 prevail US trial in patients with primary hyperlipidemia or mixed dyslipidemia. Journal of the American College of Cardiology 2012;59(13 Suppl 1):E1692. [EMBASE: 70715132]
-
- Miller PE, Martin SS, Joshi PH, Jones SR, Massaro JM, D'Agostino RB, et al. Pitavastatin 4 mg provides significantly greater reduction in remnant lipoprotein cholesterol compared with pravastatin 40 mg: results from the short-term phase IV Prevail-US trial in patients with primary hyperlipidemia or mixed dyslipidemia. Clinical Therapeutics 2016;38(3):603-9. [MEDLINE: ] - PubMed
-
- Morgan R, Campbell SE, Kryzhanovski VA, Yu CY, Sponseller CA, Davidson MH. Pitavastatin 4 mg is superior to pravastatin 40 mg in LDL-C reduction: results from Prevail US trial in primary hyperlipidemia or mixed dyslipidemia. Journal of Clinical Lipidology 2012;6(3):280-2. [EMBASE: 70819817]
-
- NCT01256476. Prevail-US: a study of pitavastatin 4 mg vs. pravastatin 40 mg in patients with primary hyperlipidemia or mixed dyslipidemia (Prevail-US) [A randomized, double-blind, active controlled, parallel group study of pitavastatin 4 mg vs. pravastatin 40 mg in patients with primary hyperlipidemia or mixed dyslipidemia]. clinicaltrials.gov/show/NCT01256476 (first received 8 December 2010). [NCT01256476]
-
- Sponseller CA, Morgan RE, Campbell SE, Yu CY, Davidson MH. Pitavastatin 4 mg significantly reduces LDL-P and increases HDL size compared with pravastatin 40 mg: results from Prevail US. Journal of Clinical Lipidology 2012;6(3):288-9. [EMBASE: 70819827]
Saito 2002a {published data only}
-
- Saito Y, Teramoto T, Yamada N, Itakura H, Hata Y, Nakaya N, et al. Clinical efficacy of nk-104 (pitavastatin), a new synthetic HMG-CoA reductase inhibitor, in the dose finding, double blind, three-group comparative study [Yōryō hakken, nijūmōken, 3-gun hikaku shiken ni okeru atarashī gōsei HMG - CoA kangen kōso sogai-zaidearu nk - 104 (pitabasutachin) no rinshō kōka]. Rynshouiyaku 2001;17(6):829-55.
-
- Saito Y, Yamada N, Teramoto T, Itakura H, Hata Y, Nakaya N, et al. Clinical efficacy of pitavastatin, a new 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, in patients with hyperlipidemia. Dose-finding study using the double-blind, three-group parallel comparison. Arzneimittelforschung 2002;52(4):251-5. [DOI: 10.1055/s-0031-1299888] - DOI - PubMed
Saito 2002b {published data only}
-
- Saito Y, Yamada N, Teramoto T, Itakura H, Hata Y, Nakaya N, et al. A randomized, double-blind trial comparing the efficacy and safety of pitavastatin versus pravastatin in patients with primary hypercholesterolemia. Atherosclerosis 2002;162(2):373-9 [erratum appears in Atherosclerosis. 2003 Jun;168(2):401]. [MEDLINE: ] - PubMed
Sakabe 2008 {published data only}
-
- Sakabe K, Fukuda N, Fukuda Y, Wakayama K, Nada T, Morishita S, et al. Comparisons of short- and intermediate-term effects of pitavastatin versus atorvastatin on lipid profiles, fibrinolytic parameter, and endothelial function. International Journal of Cardiology 2008;125(1):136-8. [MEDLINE: ] - PubMed
Sansanayudh 2010 {published data only}
-
- Sansanayudh N, Wongwiwatthananukit S, Putwai P, Dhumma-Upakorn R. Comparative efficacy and safety of low-dose pitavastatin versus atorvastatin in patients with hypercholesterolemia. Annals of Pharmacotherapy 2010;44(3):415-23. [MEDLINE: ] - PubMed
Sasaki 2008 {published data only}
-
- Sasaki J, Ikeda Y, Kuribayashi T, Kajiwara K, Biro S, Yamamoto K, et al. A 52-week, randomized, open-label, parallel-group comparison of the tolerability and effects of pitavastatin and atorvastatin on high-density lipoprotein cholesterol levels and glucose metabolism in Japanese patients with elevated levels of low-density lipoprotein cholesterol and glucose intolerance. Clinical Therapeutics 2008;30(6):1089-101. [MEDLINE: ] - PubMed
Shimabukuro 2011 {published data only}
Sone 2002 {published data only}
-
- Sone H, Takahashi A, Shimano H, Ishibashi S, Yoshino G, Morisaki N, et al. HMG-CoA reductase inhibitor decreases small dense low-density lipoprotein and remnant-like particle cholesterol in patients with type-2 diabetes. Life Sciences 2002;71(20):2403-12. [MEDLINE: ] - PubMed
Stender 2013 {published data only}
-
- Center for Drug Evaluation and Research. Study to compare the efficacy and safety of pitavastatin and pravastatin in elderly patients [Study of pitavastatin 1 mg vs. pravastatin 10 mg, pitavastatin 2 mg vs. pravastatin 20 mg and pitavastatin 4 mg vs pravastatin 40 mg ( following up-titration) in elderly subjects with primary hypercholesterolemia or combined dyslipidemia [NK-104-306]]. FDA Open Trials/show/NK-104-306 (first received 1 October 2008). [www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022363s000_MedR_P1.pdf]
-
- EUCTR2005 001039 31. Study to compare the efficacy and safety of pitavastatin and pravastatin in elderly patients [Study of pitavastatin 1 mg vs pravastatin 10 mg, pitavastatin 2 mg vs. pravastatin 20 mg and pitavastatin 4 mg vs. pravastatin (following up-titration) in elderly patients with primary hypercholesterolemia or combined dyslipidemia]. EU Clinical Trials Register/show/EUCTR2005 001039 31 (first received 8 November 2005). [regroup-production.s3.amazonaws.com/documents/ReviewReference/45536664/E...
-
- NCT00257686. Study to compare the efficacy and safety of pitavastatin and pravastatin in elderly patients [Study Of pitavastatin 1 mg vs. pravastatin 10 mg, pitavastatin 2 mg vs. pravastatin 20 mg and pitavastatin 4 mg vs. pravastatin 40 mg ( following up-titration) in elderly patients with primary hypercholesterolemia or combined dyslipidemia]. clinicaltrials.gov/show/NCT00257686 (first received 23 November 2005). [NCT00257686]
-
- Stender S, Budinski D, Gosho M, Hounslow N. Pitavastatin shows greater lipid-lowering efficacy over 12 weeks than pravastatin in elderly patients with primary hypercholesterolaemia or combined (mixed) dyslipidaemia. European Journal of Preventive Cardiology 2013;20(1):40-53. [DOI: 10.1177/2047487312451251] - DOI - PubMed
Suzuki 2009 {published data only}
-
- Suzuki S, Hattori H, Kato K, Kanemaki K, Kimura A, Haga M, et al. Clinical results of combination therapy with pitavastatin calcium and dextran sulfate sodium in patients with mixed hyperlipidemia [Kongō kōshikesshō kanja ni okeru pitabasutachinkarushiumu to ryūsan dekisutoran'natoriumu no heiyō ryōhō no rinshō kekka]. Japanese Pharmacology and Therapeutics 2009;37(7):587-96. [EMBASE: 2009464427]
Tateishi 2011 {published data only}
-
- Tateishi J. Efficacy of three potent statins in patients with hypercholesterolemia who were newly prescribed statins [Atarashiku shohō sa reta sutachindeatta kō koresuterōru kesshō kanja ni okeru 3 ttsu no kyōryokuna sutachin no yūkōsei]. Therapeutic Research 2011;32(12):1653-61. [EMBASE: 2012051834]
Teramoto 2001 {published data only}
-
- Teramoto T, Saito Y, Yamada N, Itakura H, Hata Y, Nakaya N, et al. Clinical efficacy and safety of NK-104 (pitavastatin), a new synthetic HMG-CoA reductase inhibitor, in the long-term treatment of hyperlipidemia [Kōshikesshō no chōki chiryō ni okeru atarashī gōsei HMG - CoA redakutāze sogai-zaidearu nk - 104 (pitabasutachin) no rinshō kōka to anzen-sei]. Rinsho Iyaku 2001;17(6):885-913.
Uzui 2014 {published data only}
-
- Uzui H, Hayashi H, Nakae I, Matsumoto T, Uenishi H, Hayasaki H, et al. Pitavastatin decreases serum lox-1 ligand levels and mt1-mmp expression in cd14-positive mononuclear cells in hypercholesterolemic patients. International Journal of Cardiology 2014;176(3):1230-2. [MEDLINE: ] - PubMed
Yamasaki 2014 {published data only}
-
- Yamasaki T, Iwashima Y, Jesmin S, Ohta Y, Kusunoki H, Hayashi S, et al. Comparison of efficacy of intensive versus mild pitavastatin therapy on lipid and inflammation biomarkers in hypertensive patients with dyslipidemia. PLOS One 2014;9(2):e89057. [DOI: 10.1371/journal.pone.0089057] - DOI - PMC - PubMed
Yanagi 2011 {published data only}
Yokote 2008 {published data only}
-
- Yokote K, Bujo H, Hanaoka H, Shinomiya M, Mikami K, Miyashita Y, et al. Multicenter collaborative randomized parallel group comparative study of pitavastatin and atorvastatin in Japanese hypercholesterolemic patients: collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (Chiba study). Atherosclerosis 2008;201(2):345-52. [DOI: 10.1016/j.atherosclerosis.2008.02.008] - DOI - PubMed
-
- Yokote K, Saito Y. Influence of statins on glucose tolerance in patients with type 2 diabetes mellitus: subanalysis of the collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (Chiba study). Journal of Atherosclerosis & Thrombosis 2009;16(3):297-8. [MEDLINE: ] - PubMed
Yoshida 2010 {published data only}
-
- Yoshida O, Kondo T, Kureishi-Bando Y, Sugiura T, Maeda K, Okumura K, et al. Pitavastatin, an HMG-CoA reductase inhibitor, ameliorates endothelial function in chronic smokers. Circulation Journal 2010;74(1):195-202 [erratum appears in Circ J. 2010 Feb;74(2):386]. [MEDLINE: ] - PubMed
Yoshida 2013 {published data only}
-
- JPRN UMIN000001783. Value of oxidant lipid lowering effect by statin intervention in hypercholesterolemia [Value of oxidant lipid lowering effect by statin intervention in hypercholesterolemia]. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN UMIN000001783 (first received 25 March 2009). [JPRN UMIN000001783]
Yoshika 2010 {published data only}
-
- Yoshika M, Komiyama Y, Masuda M, Yokoi T, Masaki H, Ohkura H, et al. Pitavastatin further decreases serum high-sensitive c-reactive protein levels in hypertensive patients with hypercholesterolemia treated with angiotensin II, type-1 receptor antagonists. Clinical & Experimental Hypertension (New York) 2010;32(6):341-6. [DOI: 10.3109/10641961003628460] - DOI - PubMed
Yoshitomi 2006 {published data only}
-
- Yoshitomi Y, Ishii T, Kaneki M, Tsujibayashi T, Sakurai S, Nagakura C, et al. Efficacy of a low dose of pitavastatin compared with atorvastatin in primary hyperlipidemia: results of a 12-week, open label study. Journal of Atherosclerosis & Thrombosis 2006;13(2):108-13. [MEDLINE: ] - PubMed
References to studies excluded from this review
Aberg 2017 {published data only}
-
- Aberg JA, Sponseller CA, Ward DJ, Kryzhanovski VA, Campbell SE, Thompson MA. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (intrepid): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV 2017;4(7):e284-94 Correction: (S2352301817300759), (10.1016/S2352-3018(17)30075-9)). [DOI: 10.1016/S2352-3018(17)30075-9] - DOI - PubMed
-
- NCT01301066. A 12-week study comparing pitavastatin 4 mg vs. pravastatin 40 mg in HIV-infected subjects [A 12-week, randomized, double-blind, active-controlled, parallel-group study comparing pitavastatin 4 mg vs. pravastatin 40 mg in HIV-infected subjects with dyslipidemia, followed by a 40-week safety extension study]. clinicaltrials.gov/show/NCT01301066 (first received December 2010). [NCT01301066]
-
- Sponseller CA, Morgan R, Campbell S, Kryzhanovski BV, Kartman C, Aberg J, et al. Pitavastatin 4 mg provides superior LDL-C reduction vs. pravastatin 40 mg over 12 weeks in HIV-infected adults with dyslipidemia, the Intrepid trial. Journal of Clinical Lipidology 2013;7(3):260. [EMBASE: 71086780]
CTRI201305003686 2013 {published data only}
-
- CTRI/2013/05/003686. Post marketing surveillance study of pitavastatin for evaluation of its safety and efficacy in patients with diabetes and high cholesterol [A post marketing surveillance study to assess the efficacy and safety of pivasta (pitavastatin) tablets in patients of type 2 diabetes mellitus and dyslipidemia]. Clinical Trials Registry - India/show/CTRI/2013/05/003686 (first received 27 May 2013). [CTRI/2013/05/003686]
CTRI201307003842 2013 {published data only}
-
- CTRI/2013/07/003842. To compare the effectiveness of two cholesterol lowering drugs in patients with high cholesterol [Efficacy of pitavastatin vs rosuvastatin in hypercholesterolemic patients - an open labeled study]. Clinical Trials Registry - India/show/CTRI/2013/07/003842 (first received 26 July 2013). [CTRI/2013/07/003842]
CTRI201309004003 2013 {published data only}
-
- CTRI/2013/09/004003. Evaluation of pitavastatin for efficacy and safety in dyslipidemic patients: a comparative randomized controlled trial [A prospective, controlled, randomized, double blind, comparative, parallel, 2-arm study to evaluate the efficacy and safety of pitavastatin (4 mg) vs. atorvastatin (20 mg) in dyslipidemic patients associated with hypertension, diabetes and coronary artery disease]. Clinical Trials Registry - India/show/CTRI/2013/09/004003 (first received 19 September 2013). [CTRI/2013/09/004003]
Horiuchi 2010 {published data only}
-
- Horiuchi Y, Hirayama S, Soda S, Seino U, Kon M, Ueno T, et al. Statin therapy reduces inflammatory markers in hypercholesterolemic patients with high baseline levels. Journal of Atherosclerosis and Thrombosis 2010;17(7):722-9. [MEDLINE: ] - PubMed
Huang 2016 {published data only}
Hyogo 2011 {published data only}
-
- Hyogo H, Ikegami T, Tokushige K, Hashimoto E, Inui K, Matsuzaki Y, et al. Efficacy of pitavastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: an open-label, pilot study. Hepatology Research 2011;41(11):1057-65. [MEDLINE: ] - PubMed
Inami 2007 {published data only}
-
- Inami N, Nomura S, Shouzu A, Omoto S, Kimura Y, Takahashi N, et al. Effects of pitavastatin on adiponectin in patients with hyperlipidemia. Pathophysiology of Haemostasis & Thrombosis 2007;36(1):1-8. [MEDLINE: ] - PubMed
Jing 2008 {published data only}
-
- Jing S, Sun NL, Wang HY, Lu XN. Efficacy and safety of pitavastatin calcium tablets in the treatment of primary hypercholesterolemia [Pǐ fá tātīng gài piàn zhìliáo yuán fā xìng gāo dǎngùchún xiě zhèng liáoxiào hé ānquán xìng 匹伐他汀钙片治疗原发性高胆固醇血症疗效和安全性]. Chinese Journal of New Drugs 2008;17(9):775-7+86. [EMBASE: 2010307143]
Joshi 2017 {published data only}
-
- Joshi PH, Miller PE, Martin SS, Jones SR, Massaro JM, D'Agostino RB, et al. Greater remnant lipoprotein cholesterol reduction with pitavastatin compared with pravastatin in HIV-infected patients. AIDS 2017;31(7):965-71. [MEDLINE: ] - PubMed
JPRN JMA IIA00056 2011 {published data only}
-
- JPRN-JMA-IIA00056. The effect of pitavastatin on the lipoprotein subfraction profile and lipid metabolism. JMACCT Clinical Trials Registry/show/PRN-JMA-IIA00056 (first received 27 January 2011). [JPRN-JMA-IIA00056]
JPRN UMIN000000685 2007 {published data only}
-
- JPRN-UMIN000000685. Stanford type B aortic dissection patients with normally hypotensive medical therapy and pitavastatin treatment effect. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000000685 (first received 1 June 2007). [JPRN-UMIN000000685]
JPRN UMIN000001600 2009 {published data only}
-
- JPRN-UMIN000001600. Effect of pitavastatin on amelioration for renal function in patients with chronic kidney disease. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000000685 (first received 1 June 2007). [URL: https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R00000...]
JPRN UMIN000002507 2009 {published data only}
-
- JPRN-UMIN000002507. Effects of aggressive lipid-lowering therapy on atherosclerosis and multi-lipoprotein profiling in patients with coronary artery disease. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000002507 (first received 1 October 2009). [JPRN-UMIN000002507]
JPRN UMIN000002680 2009 {published data only}
-
- JPRN-UMIN000002680. Randomized evaluation of aggressive or moderate lipid lowering therapy with pitavastatin in coronary artery disease (Real-Cad). UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000002680 (first received 27 October 2009). [JPRN-UMIN000002680]
JPRN UMIN000003554 2010 {published data only}
-
- JPRN-UMIN000003554. Comparison of effects of pitavastatin and atorvastatin on glucose and lipid metabolism in diabetic patients with hyperlipidemia. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000003554 (first received 1 May 2010). [JPRN-UMIN000003554]
JPRN UMIN000003628 2010 {published data only}
-
- JPRN-UMIN000003628. Toyama anti-arteriosclerosis and plaque assessment trial by aggressive lipid lowering therapy with pitavastatin in peripheral artery disease. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000003628 (first received 1 June 2010). [JPRN-UMIN000003628]
JPRN UMIN000005489 2011 {published data only}
-
- JPRN-UMIN000005489. Effect of pitavastatin on the coronary endothelial dysfunction after PCI with DES (drug-eluting stent). UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000005489 (first received 22 April 2011). [JPRN-UMIN000005489]
JPRN UMIN000005501 2011 {published data only}
-
- JPRN-UMIN000005501. Effect of pitavastatin on restenosis with carotid artery stenting. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000005501 (first received 25 April 2011). [JPRN-UMIN000005501]
JPRN UMIN000007130 2012 {published data only}
-
- JPRN-UMIN000007130. A multicenter randomized study evaluating the efficacy and safety of HMG-CoA reductase inhibitors (statins) in dyslipidemic patients with nephrosclerosis. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000007130 (first received 24 January 2012). [JPRN-UMIN000007130]
JPRN UMIN000007695 2012 {published data only}
-
- JPRN-UMIN000007695. Comparison of effects of pitavastatin and rosuvastatin on patients with chronic heart failure. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000007695 (first received 10 April 2012). [URL: JPRN-UMIN000007695]
JPRN UMIN000009241 2012 {published data only}
-
- JPRN-UMIN000009241. The comparison of the effects of rosuvastatin 5 mg and pitavastatin 2 mg on hypertensive patients. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000009241 (first received 1 November 2012). [JPRN-UMIN000009241]
JPRN UMIN000013384 2014 {published data only}
-
- JPRN-UMIN000013384. Effect of pitavastatin on high density lipoprotein cholesterol levels in hyperlipidemia. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000013384 (first received 10 March 2014). [JPRN-UMIN000013384]
JPRN UMIN000019020 2015 {published data only}
-
- JPRN-UMIN000019020. Investigation of lipid-improving and pleiotrophic effects of pitavastatin. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000019020 (first received 15 September 2015). [URL: JPRN-UMIN000019020]
Kawashiri 2008 {published data only}
-
- Kawashiri MA, Nohara A, Tada H, Mori M, Tsuchida M, Katsuda S, et al. Comparison of effects of pitavastatin and atorvastatin on plasma coenzyme q10 in heterozygous familial hypercholesterolemia: results from a crossover study. Clinical Pharmacology & Therapeutics 2008;83(5):731-9. [MEDLINE: ] - PubMed
Matsubara 2012 {published data only}
-
- Matsubara T, Naruse K, Arakawa T, Nakao M, Yokoi K, Oguri M, et al. Impact of pitavastatin on high-sensitivity c-reactive protein and adiponectin in hypercholesterolemic patients with the metabolic syndrome: the Premium study. Journal of Cardiology 2012;60(5):389-94. [MEDLINE: ] - PubMed
-
- NCT00444717. Impact of pitavastatin in hypercholesterolemic patients with metabolic syndrome [Multicenter study for anti-oxidative and anti-inflammatory effects of pitavastatin in hypercholesterolemic patients with metabolic syndrome]. clinicaltrials.gov/show/NCT00444717 (first received 8 March 2007). [NCT00444717]
Minai 2008 {published data only}
-
- Minai K, Inoue Y, Miyata S, Nakae S, Azuma Y, Uehara Y, et al. Efficacy and safety of pitavastatin, HMG-CoA reductase inhibitor, in patients of coronary heart disease with hyperlipidemia. Therapeutic Research 2008;29(9):1611-9. [EMBASE: 2008509864]
Muto 2013 {published data only}
-
- Muto E. Pitavastatin ameliorates endothelial function in diabetics. Japanese Pharmacology and Therapeutics 2013;41(7):685-8. [EMBASE: 2013528826]
Nakamura 2006 {published data only}
-
- Nakamura T, Sugaya T, Kawagoe Y, Suzuki T, Inoue T, Node K. Effect of pitavastatin on urinary liver-type fatty-acid-binding protein in patients with nondiabetic mild chronic kidney disease. American Journal of Nephrology 2006;26(1):82-6. [MEDLINE: ] - PubMed
Nakaya 2001 {published data only}
-
- Nakaya N, Saito Y, Morisaki N, Tamura K, Shirai K, Shinomiya M, et al. Phase II clinical study of NK-104 (pitavastatin) in patients with hyperlipidemia. Rinsho Iyaku 2001;17(6):789-806.
NCT02670434 2016 {published data only}
-
- NCT02670434. Safety and efficacy comparison study of NK-104-CR (controlled release) in patients with primary hyperlipidemia or mixed dyslipidemia. clinicaltrials.gov/show/NCT02670434 (first received 1 February 2016). [NCT02670434]
NCT02799758 2016 {published data only}
-
- NCT02799758. Efficacy & long-term safety comparison study of NK-104-CR & livalo® IR with primary hyperlipidemia or mixed dyslipidemia. clinicaltrials.gov/show/NCT02799758 (first received 15 June 2016). [NCT02799758]
Nishiguchi 2018 {published data only}
-
- JPRN-UMIN000002526. Effects of statin on renal function in patients with chronic kidney disease. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000002526 (first received 18 September 2009). [JPRN-UMIN000002526]
-
- JPRN-UMIN000002678. Effect of pitavastatin on coronary fibrous-cap thickness - assessment by fourier-domain optical coherence tomography (Escort). UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000002678 (first received 1 November 2009). [JPRN-UMIN000002678]
-
- Nishiguchi T, Kubo T, Tanimoto T, Ino Y, Matsuo Y, Yamano T, et al. Effect of early pitavastatin therapy on coronary fibrous-cap thickness assessed by optical coherence tomography in patients with acute coronary syndrome: the Escort study. Journal of the American College of Cardiology: Cardiovascular Imaging 2018;11(6):829-38. [MEDLINE: ] - PubMed
Nomura 2008 {published data only}
-
- Nomura S, Shouzu A, Omoto S, Inami N, Tanaka A, Nanba M, et al. Correlation between adiponectin and reduction of cell adhesion molecules after pitavastatin treatment in hyperlipidemic patients with type 2 diabetes mellitus. Thrombosis Research 2008;122(1):39-45. [MEDLINE: ] - PubMed
Nozue 2015 {published data only}
Rui 2014 {published data only}
-
- Rui Y, Youyou D, Yanzhou Z, Qinghua C, Luosha Z, Ling L. Comparison of clinical efficacy of different statins on cardiovascular events following percutaneous coronary intervention. Experimental and Clinical Cardiology 2014;20(8):2598-614. [EMBASE: 2014538748]
Watanabe 2015 {published data only}
Wongprikorn 2016 {published data only}
-
- NCT02442700. Effects of pitavastatin on lipid profiles in HIV-infected patients with dyslipidemia and receiving atazanavir/ritonavir [Effects of pitavastatin on lipid profiles in HIV-infected patients with dyslipidemia and receiving atazanavir/ritonavir: a randomized, double-blind, crossover study]. clinicaltrials.gov/show/NCT02442700 (first received 13 May 2015). [NCT02442700]
-
- Wongprikorn A, Sukasem C, Puangpetch A, Numthavej P, Thakkinstian A, Kiertiburanakul S. Effects of pitavastatin on lipid profiles in HIV-infected patients with dyslipidemia and receiving atazanavir/ritonavir: a randomized, double-blind, crossover study. PLOS One [Electronic Resource] 2016;11(6):e0157531. [MEDLINE: ] - PMC - PubMed
Yagi 2011 {published data only}
-
- Yagi S, Akaike M, Aihara K, Iwase T, Ishikawa K, Yoshida S, et al. Effect of low-dose (1 mg/day) pitavastatin on left ventricular diastolic function and albuminuria in patients with hyperlipidemia. American Journal of Cardiology 2011;107(11):1644-9. [MEDLINE: ] - PubMed
Yao 2016 {published data only}
-
- Yao R, Du Y, Zhang Y, Chen Q, Zhao L, Li L. Comparison of clinical efficacy of different statins on cardiovascular events following percutaneous coronary intervention. International Journal of Clinical and Experimental Medicine 2016;9(2):4356-63. [EMBASE: 20160256110]
Zhu 2010 {published data only}
-
- Zhu H, Li D, Xia Y, Zhang Y, Xu T, Pan D, et al. Therapeutic effects of domestic pitavastatin calcium tablets and atorvastatin calcium tablets on hypercholesterolemia. Xuzhou Yixueyuan Xuebao 2010;30(11):719-21.
References to studies awaiting assessment
JPRN UMIN000003055 2010 {published data only}
-
- JPRN-UMIN000003055. Effectiveness of ezetimibe or pitavastatin calcium calcium in Nash/Nafld patients with high cholesterol levels: a randomized controlled study. UMIN Clinical Trials Registry (UMIN-CTR)/show/JPRN-UMIN000003055 (first received 18 January 2010). [JPRN-UMIN000003055]
References to ongoing studies
KCT0001730 2015 {published data only}
-
- Clinical Research Information Service. Effects of lipid lowering on endothelial function [Effects of lipid lowering on endothelial function: statin treatment for atherosclerotic vascular disease (STA study)]. cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=5808 (first received 8 December 2015). [KCT0001730 ]
NCT01402843 2011 {published data only}
-
- NCT01402843. Efficacy, safety of coadministered pitavastatin and valsartan in patients with hypertension and dyslipidemia (Coctail) [A randomized, double blind, double dummy, placebo controlled phase III trial to evaluate the efficacy, safety of coadministered pitavastatin and valsartan in patients with hypertension and dyslipidemia (Coctail study)]. clinicaltrials.gov/show/NCT01402843 (first received 26 July 2011). [NCT01402843]
NCT01710007 2011 {published data only}
-
- NCT01710007. Efficacy and safety study of pitavastatin for hypercholesterolemia [A prospective, double-blind, randomized, parallel, multiple-center study to compare the efficacy and safety of 1PC002 and atorvastatin in Taiwanese patients with hypercholesterolemia]. clinicaltrials.gov/show/NCT01710007 (first received 18 October 2012). [NCT01710007]
Additional references
Adams 2014
Adams 2015
Adams 2016
Adams 2017
Adams 2018
Adams 2020
Australian Government 2013
-
- Department of Health Therapeutic Goods Administration. Australian public assessment report for pitavastatin. www.tga.gov.au/sites/default/files/auspar-pitavastatin-130902.pdf (accessed prior to 30 May 2020).
Bandolier 2004
-
- Bandolier. Cholesterol lowering with statins. Bandolier 2004:121-2.
Boekholdt 2012
-
- Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosaJC, Nestel PJ, et al. Association of ldl cholesterol, non-hdl cholesterol, and apolipoprotein b levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA 2012;307(12):1302–9. - PubMed
CDC 2011
-
- Centers for Disease Control and Prevention (CDC). Million hearts: strategies to reduce the prevalence of leading cardiovascular disease risk factors - United States, 2011. MMWR - Morbidity & Mortality Weekly Report 2011;60(36):1248-51. [MEDLINE: ] - PubMed
Chowdhury 2009
-
- Chowdhury IN, Gortler D. Center for Drug Evaluation and Research application number: 22-363 Medical Review(s). assets.documentcloud.org/documents/3199266/NDA022363-0-Approval-Medical-... (accessed prior to 30 May 2020).
Covidence 2019 [Computer program]
-
- Veritas Health Innovation Covidence. Version accessed 1 August 2017. Melbourne, Australia: Veritas Health Innovation, 2019.Available at covidence.org.
CTT 2005
-
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al, Cholesterol Treatment Trialists (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267-78. [MEDLINE: ] - PubMed
Edwards 2003
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [MEDLINE: ] - PubMed
Gaw 2000
-
- Gaw A, Packard CJ, Shepherd J. Statins: the HMG CoA Reductase Inhibitors in Perspective. London, England: Martin Dunitz Ltd, 2000. [1853174688]
GraphPad Prism 4 [Computer program]
-
- GraphPad Software Inc. GraphPad Prism 4. Version 4.0. La Jolia: GraphPad Software Inc., 2003.
Grundy 2004
-
- Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Journal of the American College of Cardiology 2004;44(3):720–32. [MEDLINE: ] - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [MEDLINE: ] - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kellick 1997
-
- Kellick KA, Burns K, McAndrew E, Haberl E, Hook N, Ellis AK. Focus on atorvastatin: an HMG-CoA reductase inhibitor for lowering both elevated ldl cholesterol and triglycerides in hypercholesterolemic patients. Formulary 1997;32(4):352-63. [EMBASE: 1997129035]
Kreatsoulas 2010
Law 2003
Liao 2005
Moghadasian 1999
-
- Moghadasian MH. Clinical pharmacology of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Life Sciences 1999;65(13):1329–37. [MEDLINE: ] - PubMed
Moher 2009
Mukhtar 2005
-
- Mukhtar RYA, Reid J, Reckless JPD. Pitavastatin. International Journal of Clinical Practice 2005;59(2):239-52. [MEDLINE: ] - PubMed
Page 2019
-
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
RevMan 2020 [Computer program]
-
- Nordic Cochrane Centre, the Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, the Cochrane Collaboration, 2020.
Roger 2011
Schaefer 2004
-
- Schaefer EJ, McNamara JR, Tayler T, Daly JA, Gleason JL, Seman LJ, et al. Comparisons of effects of statins (atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin) on fasting and postprandial lipoproteins in patients with coronary heart disease versus control subjects. American Journal of Cardiology 2004;93(1):31-9. [MEDLINE: ] - PubMed
Schectman 1996
-
- Schectman G, Hiatt J. Dose-response characteristics of cholesterol-lowering drug therapies: implications for treatment. Annals of Internal Medicine 1996;125(12):990-1000. [MEDLINE: ] - PubMed
Schünemann 2019a
-
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Schünemann 2019b
-
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Teramoto 2009
-
- Teramoto T, Shimano H, Yokote K, Urashima M. Effects of pitavastatin (livalo tablet) on high density lipoprotein cholesterol (hdl-c) in hypercholesterolemia. Journal of Atherosclerosis and Thrombosis 2009;16(5):654-61. [MEDLINE: ] - PubMed
Thompson 2005
-
- Thompson JF, Man M, Johnson KJ, Wood LS, Lira ME, Lloyd DB, et al. An association study of 43 SNPs in 16 candidate genes with atorvastatin response. Pharmacogenomics Journal 2005;5(6):352-8. [PMID: ] - PubMed
Tsang 2002
-
- Tsang MB, Adams SP, Jauca C, Wright JM. In some systematic reviews placebos may not be necessary: an example from a statin dose-response study. In: 10th Cochrane Colloquium; 2002 31 July - August 2002; Stavanger, Norway. Vol. Abstracts. Stavanger, 2002:Poster 29.
Ward 2007
-
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technology Assessment (Winchester, England) 2007;11(14):1-160, iii-iv. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

