Generation and Reactivity of C(1)-Ammonium Enolates by Using Isothiourea Catalysis
- PMID: 32557875
- PMCID: PMC7894297
- DOI: 10.1002/chem.202002059
Generation and Reactivity of C(1)-Ammonium Enolates by Using Isothiourea Catalysis
Abstract
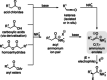
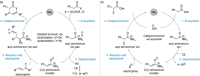
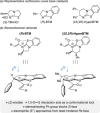
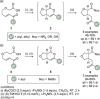
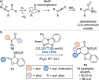
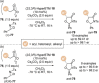
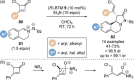
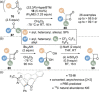
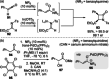
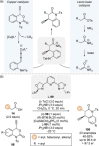
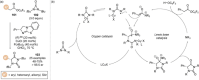
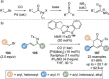
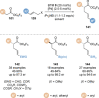
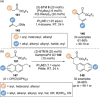
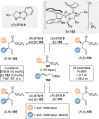
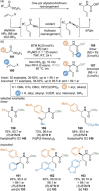
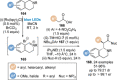
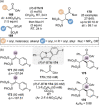
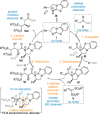
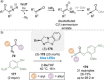
C(1)-Ammonium enolates are powerful, catalytically generated synthetic intermediates applied in the enantioselective α-functionalisation of carboxylic acid derivatives. This minireview describes the recent developments in the generation and application of C(1)-ammonium enolates from various precursors (carboxylic acids, anhydrides, acyl imidazoles, aryl esters, α-diazoketones, alkyl halides) using isothiourea Lewis base organocatalysts. Their synthetic utility in intra- and intermolecular enantioselective C-C and C-X bond forming processes on reaction with various electrophiles will be showcased utilising two distinct catalyst turnover approaches.
Keywords: C(1)-ammonium enolates; aryloxides; catalyst turnover; formal cycloaddition; isothioureas.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






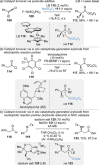
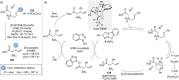

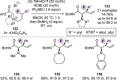

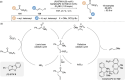

References
-
- Denmark S. E., Beutner G. L., Angew. Chem. Int. Ed. 2008, 47, 1560–1638; - PubMed
- Angew. Chem. 2008, 120, 1584–1663.
-
- None
-
- Erkkilä A., Majander I., Pihko P. M., Chem. Rev. 2007, 107, 5416–5470; - PubMed
-
- Mukherjee S., Yang J. W., Hoffmann S., List B., Chem. Rev. 2007, 107, 5471–5569; - PubMed
-
- Mečiarová M., Tivoský P., Šebesta R., New J. Chem. 2016, 40, 4855–4864.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

