Biocatalysis: Enzymatic Synthesis for Industrial Applications
- PMID: 32558088
- PMCID: PMC7818486
- DOI: 10.1002/anie.202006648
Biocatalysis: Enzymatic Synthesis for Industrial Applications
Abstract
Biocatalysis has found numerous applications in various fields as an alternative to chemical catalysis. The use of enzymes in organic synthesis, especially to make chiral compounds for pharmaceuticals as well for the flavors and fragrance industry, are the most prominent examples. In addition, biocatalysts are used on a large scale to make specialty and even bulk chemicals. This review intends to give illustrative examples in this field with a special focus on scalable chemical production using enzymes. It also discusses the opportunities and limitations of enzymatic syntheses using distinct examples and provides an outlook on emerging enzyme classes.
Keywords: biocatalysis; enzyme catalysis; industrial catalysis; organic synthesis; stereoselectivity.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
R.S. is an employee of Novartis, J.C.M. is an employee of Merck & Co, Inc. All have been involved in inventions cited in this review. K.B. declares to own stock of several of the companies mentioned in the review.
Figures

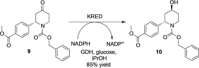







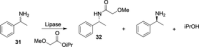





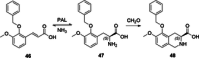








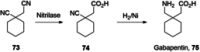

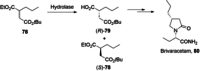



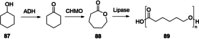
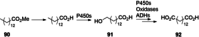
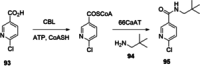






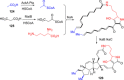






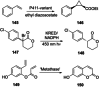
References
-
- None
-
- Bornscheuer U. T., Huisman G. W., Kazlauskas R. J., Lutz S., Moore J. C., Robins K., Nature 2012, 485, 185–194; - PubMed
-
- Sheldon R. A., Brady D., ChemSusChem 2019, 12, 2859–2881; - PubMed
-
- Fryszkowska A., Devine P. N., Curr. Opin. Chem. Biol. 2020, 55, 151–160; - PubMed
-
- Sheldon R. A., Woodley J. M., Chem. Rev. 2018, 118, 801–838; - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

