Fast intrinsic optical signal correlates with activation phase of phototransduction in retinal photoreceptors
- PMID: 32558598
- PMCID: PMC7400727
- DOI: 10.1177/1535370220935406
Fast intrinsic optical signal correlates with activation phase of phototransduction in retinal photoreceptors
Abstract
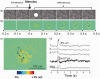
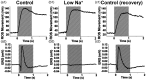
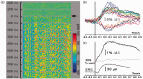
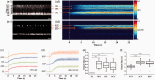
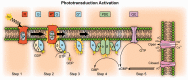
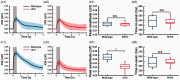
As the center of phototransduction, retinal photoreceptors are responsible for capturing and converting photon energy to bioelectric signals for following visual information processing in the retina. This article summarizes experimental observation and discusses biophysical mechanism of fast photoreceptor-intrinsic optical signal (IOS) correlated with early phase of phototransduction. Quantitative imaging of fast photoreceptor-IOS may provide objective optoretinography to advance the study and diagnosis of age-related macular degeneration, retinitis pigmentosa, diabetic retinopathy, and other eye diseases that can cause photoreceptor dysfunctions.
Keywords: Optoretinography; age-related macular degeneration; diabetic retinopathy; intrinsic optical signal; optical coherence tomography; optophysiology; photoreceptor; phototransduction; retinitis pigmentosa.
Figures



Similar articles
-
In vivo optoretinography of phototransduction activation and energy metabolism in retinal photoreceptors.J Biophotonics. 2021 May;14(5):e202000462. doi: 10.1002/jbio.202000462. Epub 2021 Feb 18. J Biophotonics. 2021. PMID: 33547871 Free PMC article.
-
Functional intrinsic optical signal imaging for objective optoretinography of human photoreceptors.Exp Biol Med (Maywood). 2021 Mar;246(6):639-643. doi: 10.1177/1535370220978898. Epub 2020 Dec 13. Exp Biol Med (Maywood). 2021. PMID: 33307802 Free PMC article.
-
Functional optical coherence tomography of retinal photoreceptors.Exp Biol Med (Maywood). 2018 Dec;243(17-18):1256-1264. doi: 10.1177/1535370218816517. Epub 2018 Nov 27. Exp Biol Med (Maywood). 2018. PMID: 30482040 Free PMC article. Review.
-
Intrinsic signal optoretinography of dark adaptation kinetics.Sci Rep. 2022 Feb 15;12(1):2475. doi: 10.1038/s41598-022-06562-4. Sci Rep. 2022. PMID: 35169239 Free PMC article.
-
Retinal remodeling in human retinitis pigmentosa.Exp Eye Res. 2016 Sep;150:149-65. doi: 10.1016/j.exer.2016.03.018. Epub 2016 Mar 26. Exp Eye Res. 2016. PMID: 27020758 Free PMC article. Review.
Cited by
-
Ultrasound Flow Imaging Study on Rat Brain with Ultrasound and Light Stimulations.Bioengineering (Basel). 2024 Feb 10;11(2):174. doi: 10.3390/bioengineering11020174. Bioengineering (Basel). 2024. PMID: 38391660 Free PMC article.
-
Challenges Associated With Ellipsoid Zone Intensity Measurements Using Optical Coherence Tomography.Transl Vis Sci Technol. 2021 Oct 4;10(12):27. doi: 10.1167/tvst.10.12.27. Transl Vis Sci Technol. 2021. PMID: 34665233 Free PMC article.
-
Toward a clinical optoretinogram: a review of noninvasive, optical tests of retinal neural function.Ann Transl Med. 2021 Aug;9(15):1270. doi: 10.21037/atm-20-6440. Ann Transl Med. 2021. PMID: 34532407 Free PMC article. Review.
-
Interpretation of anatomic correlates of outer retinal bands in optical coherence tomography.Exp Biol Med (Maywood). 2021 Oct;246(20):2140-2150. doi: 10.1177/15353702211022674. Epub 2021 Jun 10. Exp Biol Med (Maywood). 2021. PMID: 34111984 Free PMC article. Review.
-
Functional Optical Coherence Tomography for Intrinsic Signal Optoretinography: Recent Developments and Deployment Challenges.Front Med (Lausanne). 2022 Apr 4;9:864824. doi: 10.3389/fmed.2022.864824. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35445037 Free PMC article. Review.
References
-
- Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci 1996; 37:1236–49 - PubMed
-
- Yang S, Zuo C, Xiao H, Mi L, Luo G, Xu X, Liu X. Photoreceptor dysfunction in early and intermediate age-related macular degeneration assessed with mfERG and spectral domain OCT. Doc Ophthalmol 2016; 132:17–26 - PubMed
-
- Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev 2002; 1:381–96 - PubMed
-
- Berson EL, Gouras P, Gunkel RD. Rod responses in retinitis pigmentosa, dominantly inherited. Arch Ophthalmol 1968; 80:58–67 - PubMed
-
- Berson EL, Goldstein EB. Early receptor potential in dominantly inherited retinitis pigmentosa. Arch Ophthalmol 1970; 83:412–20 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

