The NLRP1 and CARD8 inflammasomes
- PMID: 32558991
- PMCID: PMC7483925
- DOI: 10.1111/imr.12884
The NLRP1 and CARD8 inflammasomes
Abstract
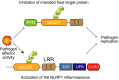
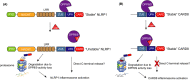
Inflammasomes are multiprotein complexes that activate inflammatory cytokines and induce pyroptosis in response to intracellular danger-associated signals. NLRP1 and CARD8 are related germline-encoded pattern recognition receptors that form inflammasomes, but their activation mechanisms and biological purposes have not yet been fully established. Both NLRP1 and CARD8 undergo post-translational autoproteolysis to generate two non-covalently associated polypeptide chains. NLRP1 and CARD8 activators induce the proteasome-mediated destruction of the N-terminal fragment, liberating the C-terminal fragment to form an inflammasome. Here, we review the danger-associated stimuli that have been reported to activate NLRP1 and/or CARD8, including anthrax lethal toxin, Toxoplasma gondii, Shigella flexneri and the small molecule DPP8/9 inhibitor Val-boroPro, focusing on recent mechanistic insights and highlighting unresolved questions. In addition, we discuss the recently identified disease-associated mutations in NLRP1 and CARD8, the potential role that DPP9's protein structure plays in inflammasome regulation, and the emerging link between NLRP1 and metabolism. Finally, we summarize all of this latest research and consider the possible biological purposes of these enigmatic inflammasomes.
Keywords: CARD8; DPP8/9; NLRP1; Val-boroPro; anthrax lethal toxin; inflammasome; proteasome; pyroptosis.
© 2020 The Authors. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197‐216. - PubMed
-
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013‐1022. - PubMed
-
- Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407‐420. - PubMed
-
- Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660‐665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

