Two ways of epigenetic silencing of TFPI2 in cervical cancer
- PMID: 32559232
- PMCID: PMC7304613
- DOI: 10.1371/journal.pone.0234873
Two ways of epigenetic silencing of TFPI2 in cervical cancer
Abstract
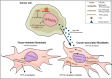
Objective: Comparison of human mRNA microarray results from tumor-associated and normal cervical fibroblasts revealed significant TFPI2 downregulation in tumor-associated fibroblasts isolated from cervical cancer, indicating that TFPI2 downregulation may play an important role in the pathogenesis of the disease. In the present work, we investigated the mechanism of TFPI2 downregulation in tumor-associated fibroblasts and tumor cells.
Methods: In vitro models of monocultures and co-cultures were established with tumor cells and fibroblasts to explore the changes of TFPI-2 expression and epigenetic modifications of the TFPI2 gene.
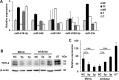
Results: The TFPI2 gene was hypermethylated only in tumor cells. Reduction of TFPI-2 protein levels in tumor-associated fibroblasts, although the gene was not methylated, suggested alternative regulatory mechanisms of gene expression, such as inhibition by microRNAs. The expression pattern of miR-23a, a gene thought to inhibit TFPI2 translation, showed changes strongly correlated to detected TFPI-2 protein alterations. Transfections with miR-23a mimics resulted in a decrease of TFPI-2 protein expression whereas miR-23a inhibitors increased the TFPI-2 amount. Due to downregulation of miR-23a expression by HPV in cancer cells, TFPI2 was silenced by promoter methylation. In contrary, miR-23a was active in HPV-free fibroblasts and inactivated TFPI2.
Conclusion: These results indicate dual epigenetic inhibition of TFPI2 on the transcription level by promoter methylation in cancer cells and on the translation level by miR-23a in tumor-associated fibroblasts. As a consequence, inactivation of the TFPI2 gene plays a strategic role in the progression of cervical cancer.
Conflict of interest statement
The employment of author (ZP) at by Maternity Obstetrics and Gynecology Private Clinic does not alter our adherence to PLOS ONE policies on sharing data and materials. The remaining authors have no competing interests to disclose.
Figures






References
-
- Fullar A, Kovalszky I, Bitsche M, Romani A, Schartinger VH, Sprinzl GM, et al. Tumor cell and carcinoma-associated fibroblast interaction regulates matrix metalloproteinases and their inhibitors in oral squamous cell carcinoma. Exp Cell Res. 2012;318(13):1517–27. Epub 2012/04/21. 10.1016/j.yexcr.2012.03.023 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

