The Epidemiology of Severe Acute Respiratory Syndrome Coronavirus 2 in a Pediatric Healthcare Network in the United States
- PMID: 32559282
- PMCID: PMC7337783
- DOI: 10.1093/jpids/piaa074
The Epidemiology of Severe Acute Respiratory Syndrome Coronavirus 2 in a Pediatric Healthcare Network in the United States
Abstract
Background: Understanding the prevalence and clinical presentation of coronavirus disease 2019 in pediatric patients can help healthcare providers and systems prepare and respond to this emerging pandemic.
Methods: This was a retrospective case series of patients tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) across a pediatric healthcare network, including clinical features and outcomes of those with positive test results.
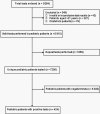
Results: Of 7256 unique children tested for SARS-CoV-2, 424 (5.8%) tested positive. Patients aged 18-21 years had the highest test positive rate (11.2%), while those aged 1-5 years had the lowest (3.9%). By race, 10.6% (226/2132) of black children tested positive vs 3.3% (117/3592) of white children. By indication for testing, 21.1% (371/1756) of patients with reported exposures or clinical symptoms tested positive vs 3.8% (53/1410) of those undergoing preprocedural or preadmission testing. Of 424 patients who tested positive for SARS-CoV-2, 182 (42.9%) had no comorbidities, 87 (20.5%) had asthma, and 55 (13.0%) were obese. Overall, 52.1% had cough, 51.2% fever, and 14.6% shortness of breath. Seventy-seven (18.2%) SARS-CoV-2-positive patients were hospitalized, of whom 24 (31.2%) required respiratory support. SARS-CoV-2-targeted antiviral therapy was given to 9 patients, and immunomodulatory therapy to 18 patients. Twelve (2.8%) SARS-CoV-2-positive patients required mechanical ventilation, and 2 patients required extracorporeal membrane oxygenation. Two patients died.
Conclusions: In this large cohort of pediatric patients tested for SARS-CoV-2, the rate of infection was low but varied by testing indication. The majority of cases were mild and few children had critical illness.
Keywords: COVID-19; coronavirus; epidemiology; testing.
© The Author(s) 2020. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- World Health Organization. Coronavirus disease (COVID-19) pandemic. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed May 1, 2020.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous