Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities
- PMID: 32559419
- PMCID: PMC7834476
- DOI: 10.1016/S2213-2600(20)30267-8
Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities
Abstract
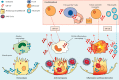
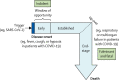
The COVID-19 pandemic is a global public health crisis, with considerable mortality and morbidity exerting pressure on health-care resources, including critical care. An excessive host inflammatory response in a subgroup of patients with severe COVID-19 might contribute to the development of acute respiratory distress syndrome (ARDS) and multiorgan failure. Timely therapeutic intervention with immunomodulation in patients with hyperinflammation could prevent disease progression to ARDS and obviate the need for invasive ventilation. Granulocyte macrophage colony-stimulating factor (GM-CSF) is an immunoregulatory cytokine with a pivotal role in initiation and perpetuation of inflammatory diseases. GM-CSF could link T-cell-driven acute pulmonary inflammation with an autocrine, self-amplifying cytokine loop leading to monocyte and macrophage activation. This axis has been targeted in cytokine storm syndromes and chronic inflammatory disorders. Here, we consider the scientific rationale for therapeutic targeting of GM-CSF in COVID-19-associated hyperinflammation. Since GM-CSF also has a key role in homoeostasis and host defence, we discuss potential risks associated with inhibition of GM-CSF in the context of viral infection and the challenges of doing clinical trials in this setting, highlighting in particular the need for a patient risk-stratification algorithm.
Trial registration: ClinicalTrials.gov NCT04326920.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
References
-
- WHO Coronavirus disease 2019 (COVID-19): situation report—142. June 10, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Bloomberg FDA approves emergency IND use of Humanigen's lenzilumab for compassionate use in COVID-19 patients. April 2, 2020. https://www.bloomberg.com/press-releases/2020-04-02/fda-approves-emergen...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

