Human Stem Cell Resources Are an Inroad to Neandertal DNA Functions
- PMID: 32559457
- PMCID: PMC7363959
- DOI: 10.1016/j.stemcr.2020.05.018
Human Stem Cell Resources Are an Inroad to Neandertal DNA Functions
Abstract
Induced pluripotent stem cells (iPSCs) from diverse humans offer the potential to study human functional variation in controlled culture environments. A portion of this variation originates from an ancient admixture between modern humans and Neandertals, which introduced alleles that left a phenotypic legacy on individual humans today. Here, we show that a large iPSC repository harbors extensive Neandertal DNA, including alleles that contribute to human phenotypes and diseases, encode hundreds of amino acid changes, and alter gene expression in specific tissues. We provide a database of the inferred introgressed Neandertal alleles for each individual iPSC line, together with the annotation of the predicted functional variants. We also show that transcriptomic data from organoids generated from iPSCs can be used to track Neandertal-derived RNA over developmental processes. Human iPSC resources provide an opportunity to experimentally explore Neandertal DNA function and its contribution to present-day phenotypes, and potentially study Neandertal traits.
Keywords: Neandertal genomics; archaic introgression; cerebral organoids; induced pluripotent stem cells; single-cell transcriptomics.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

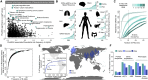

Comment in
-
Neanderthal-like 'mini-brains' created in lab with CRISPR.Nature. 2021 Feb;590(7846):376-377. doi: 10.1038/d41586-021-00388-2. Nature. 2021. PMID: 33574596 No abstract available.
References
-
- Angerer P., Haghverdi L., Büttner M., Theis F.J., Marr C., Buettner F. destiny: diffusion maps for large-scale single-cell data in R. Bioinformatics. 2016;32:1241–1243. - PubMed
-
- Bonder M.J., Smail C., Gloudemans M.J., Frésard L., Jakubosky D., D’Antonio M., Li X., Ferraro N.M., Carcamo-Orive I., Mirauta B. Systematic assessment of regulatory effects of human disease variants in pluripotent cells. bioRxiv. 2019 doi: 10.1101/784967. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

