Arterial spin labeling detects perfusion patterns related to motor symptoms in Parkinson's disease
- PMID: 32559629
- PMCID: PMC7554132
- DOI: 10.1016/j.parkreldis.2020.05.014
Arterial spin labeling detects perfusion patterns related to motor symptoms in Parkinson's disease
Abstract
Introduction: Imaging neurovascular disturbances in Parkinson's disease (PD) is an excellent measure of disease severity. Indeed, a disease-specific regional pattern of abnormal metabolism has been identified using positron emission tomography. Only a handful of studies, however, have applied perfusion MRI to detect this disease pattern. Our goal was to replicate the evaluation of a PD-related perfusion pattern using scaled subprofile modeling/principal component analysis (SSM-PCA).
Methods: We applied arterial spin labeling (ASL) MRI for this purpose. Uniquely, we assessed this pattern separately in PD individuals ON and OFF dopamine medications. We further compared the existence of these patterns and their strength in each individual with their Movement Disorder Society-Unified Parkinson's Disease Rating Scale motor (MDS-UPDRS) scores, cholinergic tone as indexed by short-term afferent inhibition (SAI), and other neuropsychiatric tests.
Results: We observed a PD-related perfusion pattern that was similar to previous studies. The patterns were observed in both ON and OFF states but only the pattern in the OFF condition could significantly (AUC=0.72) differentiate between PD and healthy subjects. In the ON condition, PD subjects were similar to controls from a CBF standpoint (AUC=0.45). The OFF pattern prominently included the posterior cingulate, precentral region, precuneus, and the subcallosal cortex. Individual principal components from the ON and OFF states were strongly associated with MDS-UPDRS scores, SAI amplitude and latency.
Conclusion: Using ASL, our study identified patterns of abnormal perfusion in PD and were associated with disease symptoms.
Keywords: Arterial spin labeling; PDRP; Parkinson's disease; UPDRS.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
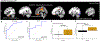
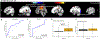



References
-
- Hammond C, Bergman H, Brown P, Pathological synchronization in Parkinson’s disease: networks, models and treatments, Trends Neurosci. 30 (2007) 357–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

