EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration
- PMID: 32560057
- PMCID: PMC7349630
- DOI: 10.3390/ijms21124271
EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration
Abstract
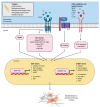
Epithelial-mesenchymal transition (EMT) and endothelial-mesenchymal transition (EndMT) are physiological processes required for normal embryogenesis. However, these processes can be hijacked in pathological conditions to facilitate tissue fibrosis and cancer metastasis. In the eye, EMT and EndMT play key roles in the pathogenesis of subretinal fibrosis, the end-stage of age-related macular degeneration (AMD) that leads to profound and permanent vision loss. Predominant in subretinal fibrotic lesions are matrix-producing mesenchymal cells believed to originate from the retinal pigment epithelium (RPE) and/or choroidal endothelial cells (CECs) through EMT and EndMT, respectively. Recent evidence suggests that EMT of RPE may also be implicated during the early stages of AMD. Transforming growth factor-beta (TGFβ) is a key cytokine orchestrating both EMT and EndMT. Investigations in the molecular mechanisms underpinning EMT and EndMT in AMD have implicated a myriad of contributing factors including signaling pathways, extracellular matrix remodelling, oxidative stress, inflammation, autophagy, metabolism and mitochondrial dysfunction. Questions arise as to differences in the mesenchymal cells derived from these two processes and their distinct mechanistic contributions to the pathogenesis of AMD. Detailed discussion on the AMD microenvironment highlights the synergistic interactions between RPE and CECs that may augment the EMT and EndMT processes in vivo. Understanding the differential regulatory networks of EMT and EndMT and their contributions to both the dry and wet forms of AMD can aid the development of therapeutic strategies targeting both RPE and CECs to potentially reverse the aberrant cellular transdifferentiation processes, regenerate the retina and thus restore vision.
Keywords: age-related macular degeneration; endothelial–mesenchymal transition; epithelial–mesenchymal transition; subretinal fibrosis; transforming growth factor-beta.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sun J.X., Chang T.F., Li M.H., Sun L.J., Yan X.C., Yang Z.Y., Liu Y., Xu W.Q., Lv Y., Su J.B., et al. SNAI1, an endothelial-mesenchymal transition transcription factor, promotes the early phase of ocular neovascularization. Angiogenesis. 2018;21:635–652. doi: 10.1007/s10456-018-9614-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

